Figures & data
TABLE 1 Dry powder inhaler and formulations used in the present study
FIG. 1. Emitted powder mass measurement set-up with a customized emitted dose powder collector for Device A and using DUSA tube for Devices B and C.
FIG. 2. Cross-sectional view of the idealized mouth-throat model with an inhaler and filter housing attached upstream and downstream, respectively.
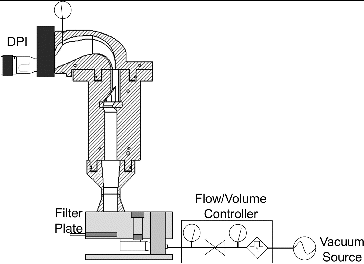
TABLE 2 Stage cut-off diameters for NGI stage 3 at test conditions
TABLE 3 Bulk powder properties of spray-dried PulmoSphere™ formulations
FIG. 3. Three particle morphologies analyzed by scanning electron microscopy: (a) spray-dried PulmoSphere™ (Batch A), (b) traditional lactose-blend with tiotropium bromide, and (c) spheronized mometasone furoate.

FIG. 4. Effect of tapped density and primary particle size on in vitro aerosol performance: (a) tapped density as function of emitted powder mass (N = 10) and (b) primary particle size, X50, as function of fine particle mass (N = 3) accumulated mass from stage 3 to MOC.
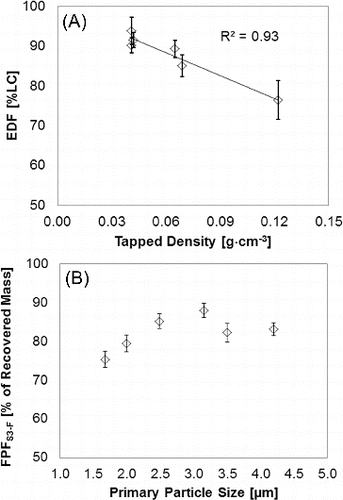
TABLE 4 NGI analysis data and mean
TABLE 5 ARLA calculator selection input parameters, all others set to default mode
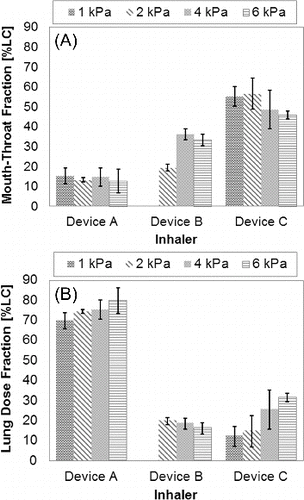
FIG. 6. In vitro lung dose performance as measured from the mouth-throat model for PulmoSpheres™ delivered by Device A as a function of mean primary particle size, tested at 1–6 kPa inhaler pressure drops.
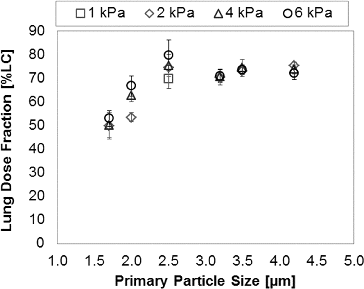
TABLE 6 Mean and standard deviation of in vitro aerosol delivered dose for different formulation platforms