Figures & data
Figure 1. A schematic of the experimental setup, including the peristaltic pump, nebulizer with N2 tank, and single particle soot photometer.
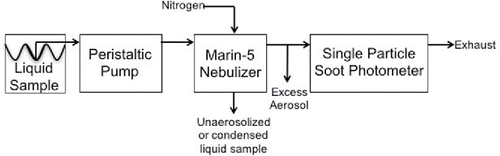
Figure 2. Absolute nebulization efficiency of the Marin-5 for flow rates (top axis) and pressures (bottom axis) applied to the gas inlet line. Data were acquired using a prepared 16 ng g−1 rBC sample, peristaltic pump rate of cc s−1, and an SP2 flow rate between 2 and 2.3 cc s−1. MicroMist 500 tip is shown in both open (2015) and closed circles (2016). MicroMist 250 tip is shown in stars.
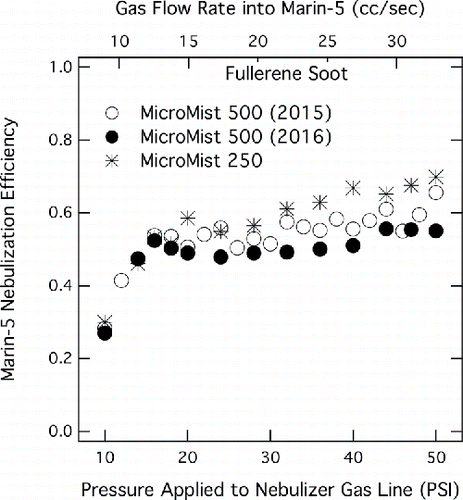
Figure 3. Absolute nebulization efficiency of the Marin-5 (with MicroMist 500) for different peristaltic pump speeds, using samples of both pure and isopropyl-doped rBC (ratio of original sample to alcohol shown in legend). Open shapes are efficiencies, and closed shapes represent the normalized signal-per-second seen by the SP2. Each curve of signal per unit time is normalized to the signal rate measured by the SP2 for the specified doping ratio at a peristaltic pump rate of cc s−1.
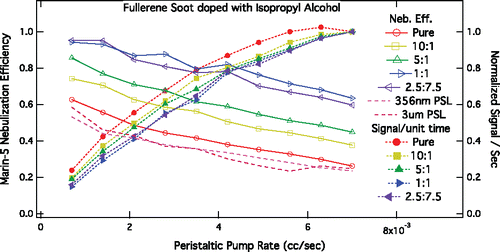
Figure 4. Measured rBC mass detection efficiency for several different SP2 sample flows and currents. Data are normalized to the average of measured MMR at 2 cc s−1 sample flow at three different laser currents.
Figure 5. PSL scatter amplitudes for 220 nm (left data set) and 505 nm (right data set) at various SP2 sample flows. As SP2 sample flow increases, PSL peaks broaden. This effect appears to be enhanced for larger particles (i.e., 505 nm vs. 220 nm).
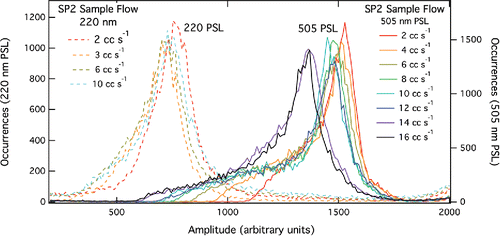
Figure 6. PSL scatter amplitudes for several sheath flows at high sample flow. The black curve is at standard running conditions of 2 cc s−1 SP2 sample flow and 10 cc s−1 sheath flow.
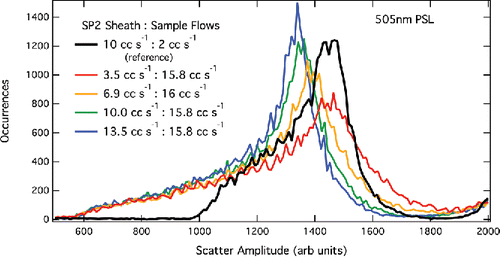
Figure 7. Measured rBC MMR for several different SP2 sheath flows and currents. MMRs are normalized to the average of measured MMRs at 2 cc s−1 sample flow and 10 cc s−1 sheath flow at three different laser currents.
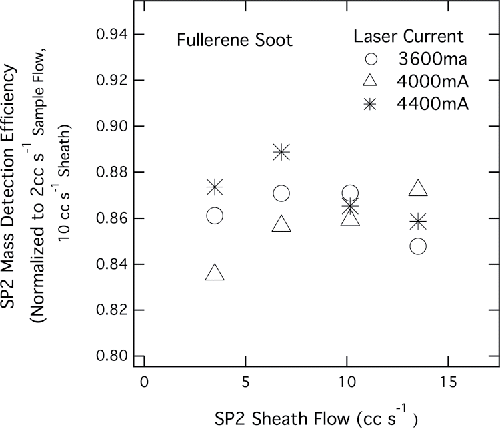
Table 1. Some examples of possible running conditions and the time needed to acquire a statistically significant size distribution, given very limited and very abundant availability of clean samples, or limited availability of dirty samples. Average particle size of 140 nm is used for calculations, with 1 ng g−1 used for clean samples and 10 ng g−1 for dirty samples. ϵuse is the fraction of total particulate in the sample that is detected by the SP2.
