Figures & data
Figure 1. The outdoor sampling site setup. (a) The real-time aerosol monitoring instruments operated each day for 1 h, including the Aerotrak Handheld Optical Particle Counter (OPC, model 9306, TSI Inc., Shoreview, MN, USA), and a DustTrak DRX Aerosol Monitor (DRX, model 8534, TSI Inc.). (b) Portable weather station (WS-2080 Wireless Weather Station, Ambient Weather, Chandler, AZ, USA). (c) Active reference samplers for bioaerosol sampling operated in 24-h intervals: three Button Aerosol Samplers (SKC Inc., Eighty Four, PA, USA) operated at 4 L/min with PTFE filters (2 µm pore size, SKC Inc.) and AirChek XR5000 Sample Pumps (SKC Inc.). (d) The passive samplers: six passive settling filters (25 mm PTFE filters with 2 µm pore size [SKC Inc.]), six REPS, and six 90 mm agar settling plates (three for culturable bacteria and three for culturable fungi).
![Figure 1. The outdoor sampling site setup. (a) The real-time aerosol monitoring instruments operated each day for 1 h, including the Aerotrak Handheld Optical Particle Counter (OPC, model 9306, TSI Inc., Shoreview, MN, USA), and a DustTrak DRX Aerosol Monitor (DRX, model 8534, TSI Inc.). (b) Portable weather station (WS-2080 Wireless Weather Station, Ambient Weather, Chandler, AZ, USA). (c) Active reference samplers for bioaerosol sampling operated in 24-h intervals: three Button Aerosol Samplers (SKC Inc., Eighty Four, PA, USA) operated at 4 L/min with PTFE filters (2 µm pore size, SKC Inc.) and AirChek XR5000 Sample Pumps (SKC Inc.). (d) The passive samplers: six passive settling filters (25 mm PTFE filters with 2 µm pore size [SKC Inc.]), six REPS, and six 90 mm agar settling plates (three for culturable bacteria and three for culturable fungi).](/cms/asset/bb241397-38f6-4d8a-b096-8e71097ce659/uast_a_1316830_f0001_b.gif)
Figure 2. Front and top views of the 3D-printed REPS film holder. Film holder diameter, X = 22 mm, and height, Y = 75 mm, are indicated in the figure as dimensions X and Y. As illustrated in the top view, one piece of polarized, ferroelectric poly(vinylidene fluoride) (PVDF) film (130 × 70 mm) is spiraled through the openings. This results in the formation of multiple layers of film with sides of opposite polarization facing each other across air channels of optimized width (2.25 mm; Therkorn et al. Citation2017).
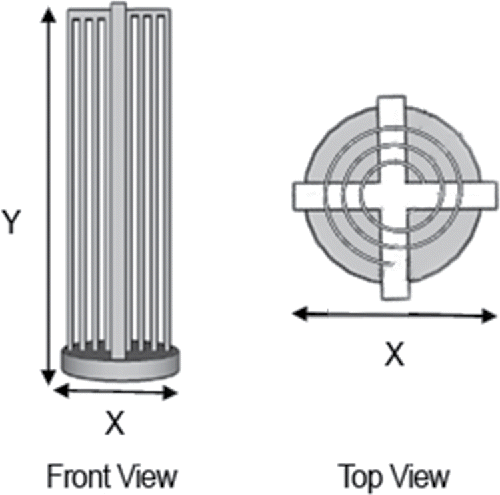
Figure 3. Relative collection efficiency (%, mean ± 1SD) of passive samplers when capturing total bioaerosol particles (culturable + non-culturable bacteria and fungi) as enumerated by Acridine Orange staining and epifluorescence microscopy. The reference Button Aerosol Samplers were operated at 4 L/min. The number of samples presented here by sampler are: Button Sampler (n = 30 per campaign), passive PTFE filter (n = 6 per campaign), and REPS (n = 6 per campaign). For the Button Sampler, three were deployed each 24-h, the average of those three was determined each 24-h, and then the campaign total was taken as the sum across the 10 days of averaged daily intervals. For the passive samplers, an average was found across the six deployed samplers for each campaign.
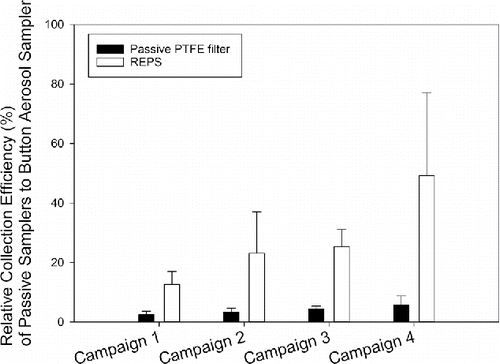
Figure 4. Relative collection efficiency (%, mean ± 1SD) of passive samplers when capturing culturable bacteria (a) and culturable fungi (b) as enumerated by CFU. The reference Button Aerosol Samplers were operated at 4 L/min. The number of samples presented here by sampler are: Button Sampler (n = 30 per campaign per microbe type), passive PTFE filter (n = 6 per campaign per microbe type), and REPS (n = 6 per campaign per microbe type). Agar spread plates were made after each 24-h Button Sampler deployment from one of the three deployed Button Samplers; the selected unit had the highest number of bacteria and fungi, as determined by microscopy, among the three Button Samplers. Campaign total was determined as the sum of average CFU across daily spread plates made in triplicate for bacteria and fungi. For the passive samplers, agar spread plates were made at the end of each campaign for the two out of six REPS and the two out of six passive PTFE filters with the highest number of stained bacteria + fungi as determined by microscopy. Campaign total was determined as the average total CFU across the spread plates made in triplicate for bacteria and fungi.
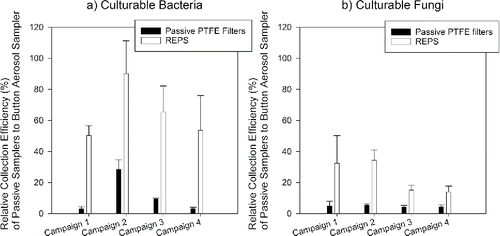
Figure 5. Average total CFU normalized to sampler's horizontal surface area (CFU/cm2, mean ± 1SD) of culturable bacteria (a) and culturable fungi (b) collected by passive samplers. For REPS, projected surface area was used (380 mm2, bottom of sampler has a 22 mm diameter). The number of samples presented here by sampler are: agar settling plates (n = 30 per campaign per microbe type), passive PTFE filter (n = 6 per campaign per microbe type), REPS (n = 6 per campaign per microbe type). Agar settling plates were deployed daily for 4 h in triplicate for bacteria and fungi. Campaign total was determined as the sum of averaged daily enumerated CFU multiplied by six to scale up to continuous 24-h sampling. For the passive samplers, agar spread plates were made at the end of each campaign for the two out of six REPS and the two out of six passive PTFE filters with the highest number of stained bacteria + fungi as determined by microscopy.
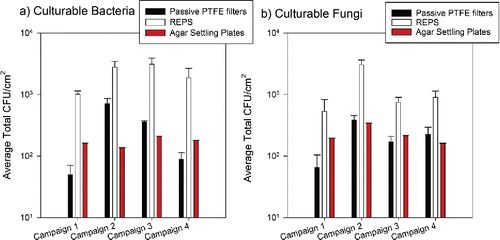
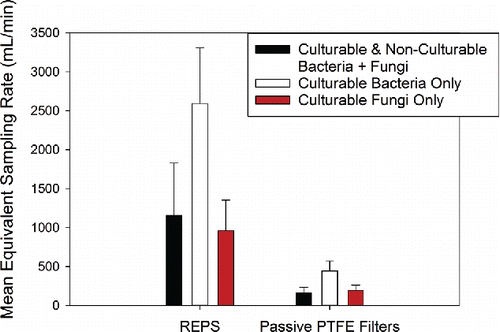
Figure 6. Average equivalent sampling flowrates (mL/min, mean ± 1SD) for passive samplers calibrated against the Button Aerosol Sampler operated at 4000 mL/min. It should be noted that, as with all calibrations of this kind, the presented equivalent sampling flowrates are likely location and sampling condition specific. The number of samples presented here by each sampler for the total collection of bacteria + fungi (culturable + non-culturable) as enumerated by Acridine Orange staining and counting by microscopy are: Button Sampler (n = 120 across campaigns 1–4), passive PTFE filter (n = 24 across campaigns 1–4), and REPS (n = 24 across campaigns 1–4). The number of samples presented here by sampler for the culturable collection of bacteria and fungi as enumerated by CFU are: Button Sampler (n = 120 across campaigns 1–4 per microbe type), passive PTFE filter (n = 24 across campaigns 1–4 per microbe type), and REPS (n = 24 across campaigns 1–4 per microbe type).