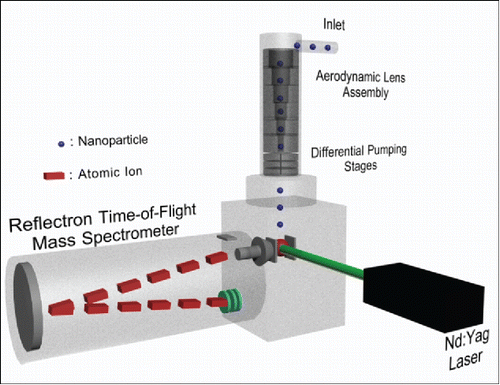
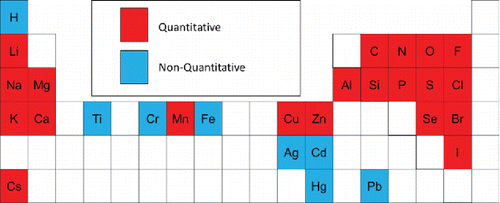
Figures & data
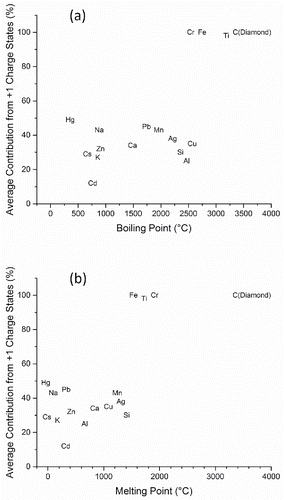
Figure 2. Summary of sucrose charge state distributions in single particle mass spectra from four monodisperse aerosol datasets, 100 spectra each. For each mass spectrum, the percentage of total signal intensity attributed to multiply charged atomic ions was calculated, and the data were sorted from highest percentage on the left to lowest percentage on the right.
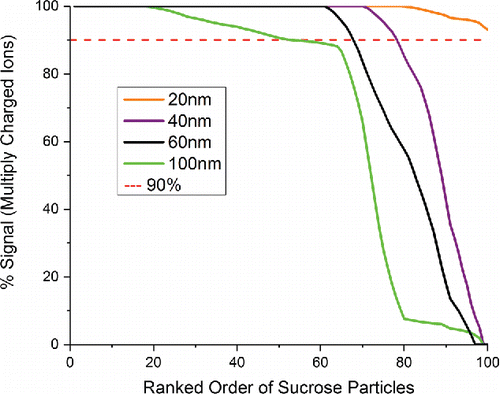
Table 1. Measured O/C ratios for monodisperse sucrose aerosols using two different quantification methods. Measurement uncertainties are reported as 1 standard deviation. (Expected O/C ratio is 0.917).
Figure 3. Average mass spectrum of 100 particles from a polydisperse aerosol composed of a 2:1 molar ratio HEPES and cesium iodide. Measurement uncertainties are reported as 1 standard deviation. Atomic ions from the various elements are marked by the bars above the spectrum.
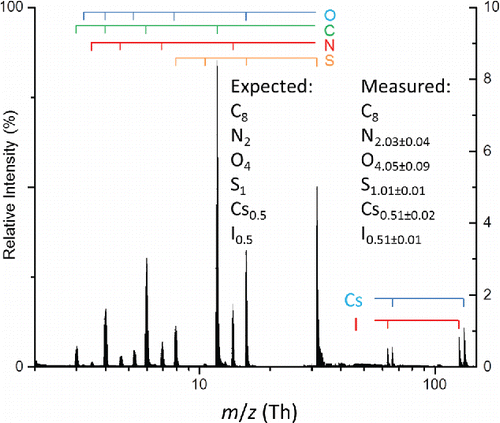
Table 2. Expected and measured elemental mole ratios from aerosols composed of HEPES and cesium iodide.
Figure 4. Average mass spectrum of 100 particles from a polydisperse aerosol composed of a 1:1 molar ratio lead(II) nitrate and HEPES. Measurement uncertainties are reported as 1 standard deviation. Atomic ions from the various elements are marked by the bars above the spectrum.
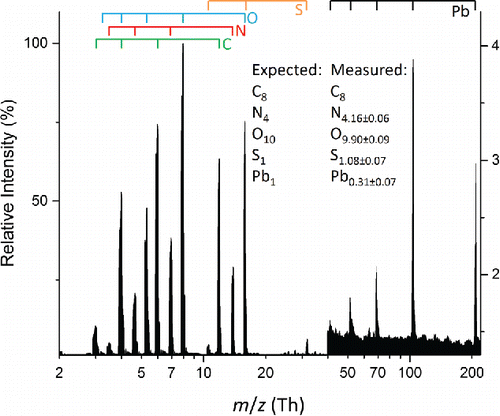
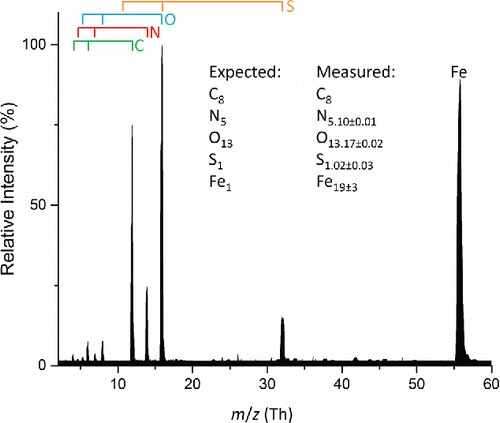
Figure 5. Average mass spectrum of 100 particles from a polydisperse aerosol composed of a 1:1 molar ratio iron(III) nitrate and HEPES. Measurement uncertainties are reported as 1 standard deviation. Atomic ions from the various elements are marked by the bars above the spectrum.