Figures & data
Table 1. Concentrations of Igepal and NaCl in initial, atomized solutions and measured corresponding r70/r80 values (mean ± standard deviation).
Figure 1. (a) Comparison of aqueous particle radii at 80% RH (r80) initially and finally for all of the aqueous particles in the evaporation and condensation experiment. (b) Comparison of NaCl aqueous particle radii at 80% RH (r80) and 70% RH (r70) calculated using Köhler theory (gray squares) and measured in the experiment (circles). The black line represents the r70/r80 ratio (0.91) for similar aqueous particles (Clegg et al. Citation1998). The gray shading shows the uncertainty associated with the RH measurements (r70/r80 of 0.86 to 0.94). In both panels, error bars represent the standard deviation of the averaged values.
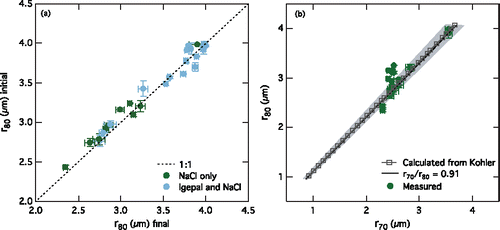
Figure 2. The median ratio of the experimental equilibrium radius at 70% RH (r70) to the equilibrium radius at 80% RH (r80) for aqueous particles containing NaCl only and NaCl with 0.4 and 4 times the CMC concentration of Igepal (Cases A and B, respectively). For aqueous particles with NaCl and 22 and 48 times the CMC concentration of Igepal (Cases C and D, respectively), the equilibrium radius at the point the aqueous particle was lost (>70% RH) is used instead. Error bars represent the standard deviation for each case. The dashed horizontal lines are at r70/r80 = 0.90 (Köhler theory) and 0.91 (Tang et al. Citation1997; Clegg et al. Citation1998). The gray shading represents the range of uncertainty around the predicted r70/r80 of 0.91 for NaCl only aqueous particles (from 0.86 to 0.94) arising from the uncertainty in the RH measurements. The overlapping open markers represent model calculated r70/r80 (Topping and McFiggans Citation2012) at the experimental molar ratios for oxalic acid (circles), succinic acid (triangles), malonic acid (diamonds), and pinolenic acid (squares).
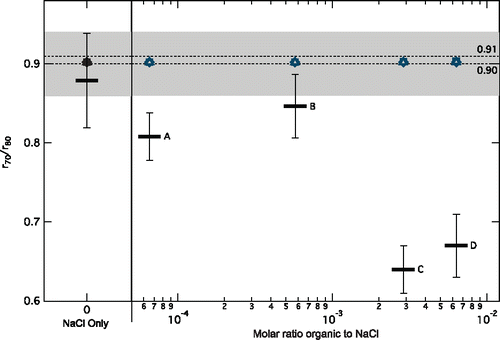
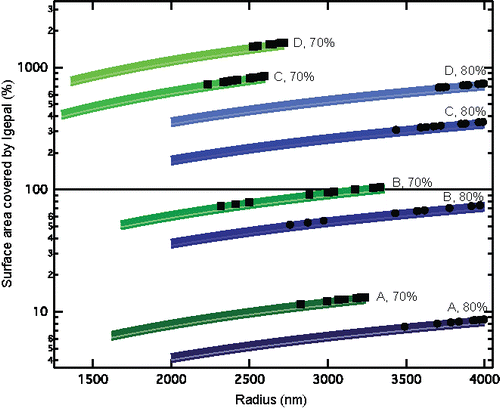
Figure 3. Percent of aqueous particle surface area covered by Igepal as a function of aqueous particle radii at 80% RH and 70% RH for the four concentrations of Igepal (Cases A to D). Thick lines are calculated as Igepal surface area of 0.357 nm2 per molecule, and shading connects to surface area of 0.32 nm2 per molecule. Individual markers represent calculated surface area coverage for measured aqueous particle radii at 80% RH (circles) and 70% RH (squares) with 0.357 nm2 per molecule.