Figures & data
Table 1. Chemical compounds tested in this study.
Figure 1. Visualization of chemical structures of the compounds in each “Group” (). A: Succinic acid, B: Malic acid, C: Oxaloacetic acid, D: Tartaric acid, E: 5-Oxoazaleic acid, F: 4-Oxoheptanedioic acid, G: 1,3-Acetonedicarboxylic acid, H: Benzene-1,3,5-Tricarboxylic acid, I: 1,3,5-Cyclohexanetricarboxylic acid, J: Tricarballylic acid, K: 1,2,3,4-Butanetetracarboxylic acid, L: Citric acid, M: Meso-Erythritol, N: Xylitol, O: Mannitol, P: Sucrose.
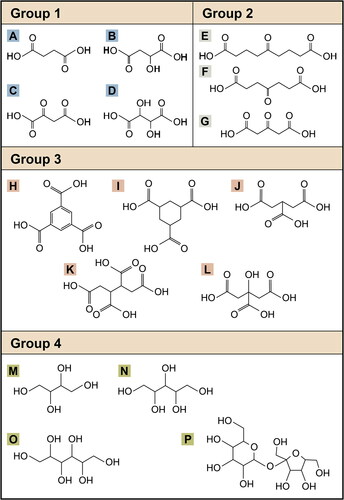
Figure 2. All compounds tested plotted onto the Van Krevelen diagram. A compound with OSC –0.5 is categorized as LO-OOA and a compound with OSC
is categorized as MO-OOA. If a compound’s OSC is –0.5 < OSC < 0, the compound can be either LO-OOA or MO-OOA and is categorized as OOA.
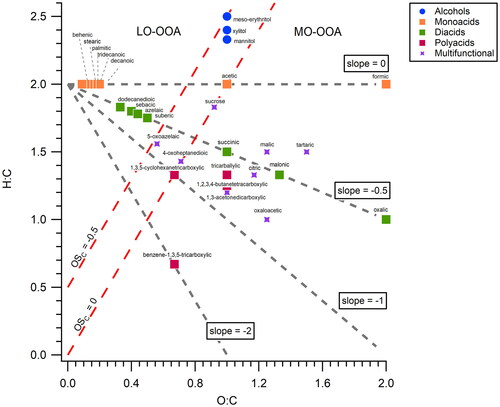
Figure 3. Fraction of main species (decarboxylation product, dehydration product, parental compound, and other) after undergoing thermal desorption for compounds in each “Type” (functional group) (). For each type, the compounds are ordered in ascending O:C ratio. For each compound, the reported fraction value is the average of two experimental measurements. Note: Only one measurement is available for sucrose.
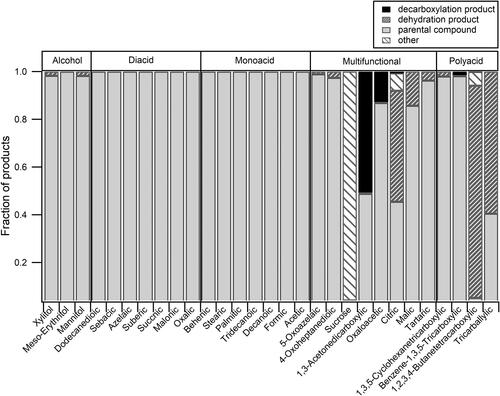
Figure 4. Measured number of carbon (measured nC), hydrogen (measured nH), and oxygen (measured nO) plotted against the true number of carbon (true nC), hydrogen (true nH), and oxygen (true nO). The solid line corresponds to a one-to-one line. The data points are color-coded according to the functional groups.
Figure 5. The scatter plots of various parameters including the nC, nO, nH, DBE, Tmax, and MW color-coded with the degree of thermal decomposition. The fraction on the y-axis indicates the fraction of parental compound left upon heating and is divided into three categories: (1) minor thermal decomposition (parental compound left: 85–100%, blue), (2) considerable thermal decomposition (parental compound left: 40–50%, purple), and (3) significant thermal decomposition (parental compound left: 0–10%, red).
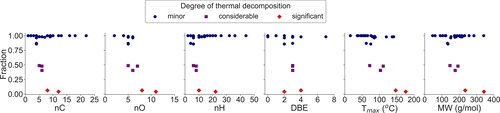
Table 2. Average effective MF and range of DBE, MW, nO, and Tmax for compounds exhibiting three different degrees of thermal decomposition: minor, considerable, and significant.
Figure 6. Fraction of major species (decarboxylated product, dehydrated product, parental compound, and other) after undergoing thermal desorption for compounds in each “Group” ( and ). For each compound, the reported fraction value is the average of two experimental measurements. Note: Only one measurement is available for sucrose.
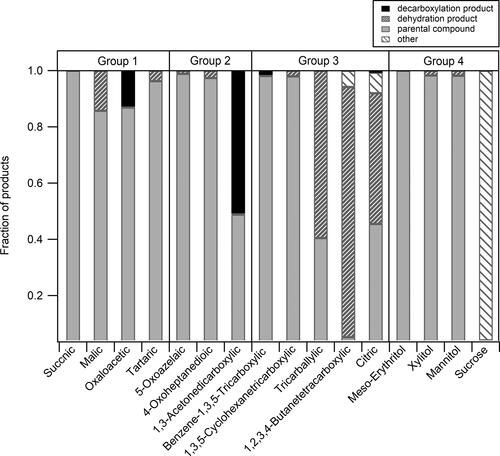
Figure 7. Fraction of parental compound detected by FIGAERO-CIMS after desorption process vs. the carbon oxidation state of polyacids. Note: succinic acid (diacid) is not classified as polyacid but added in this figure as it shows the effect of adding more acids to the diacid.
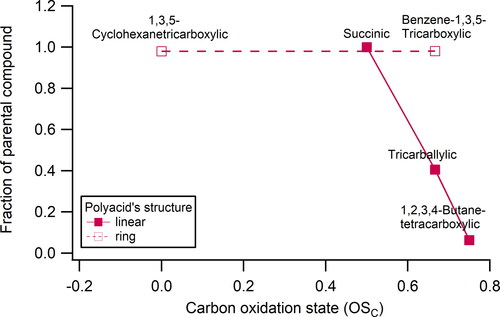
Figure 8. Van Krevelen diagrams of the average mass spectra of each OA factor resolved by PMF analysis of FIGAERO-CIMS data acquired at a field campaign in Yorkville, GA (Chen et al. Citation2020). The data points are colored by Tmax. The five factors are daytime more oxidized (Day-MO), daytime organic-nitrate-rich (Day-ONRich), morning less oxidized (MRN-LO), afternoon less oxidized (AFTN-LO), and nighttime organic-nitrate-rich (NGT-ONRich).
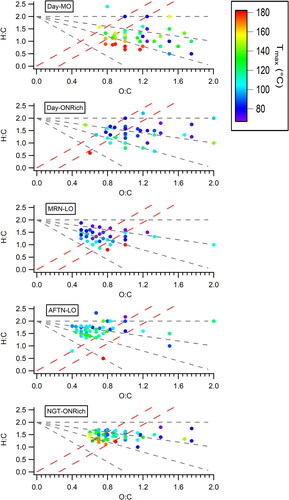
Figure 9. Fraction of four species (C6H8O7 (parental compounds denoted as M), C6H6O6 (primary dehydration product), C6H4O5 (secondary dehydration product), and C5H6O4 (decarboxylation product of primary dehydration product)) for ramping rate of 3.3 C min−1, 13.3
C min−1, and 40
C min−1.
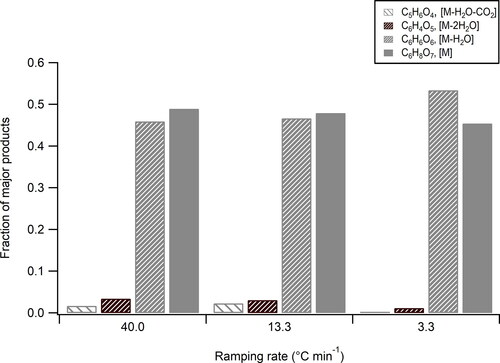
Figure 10. Thermal desorption profiles (thermograms) of citric acid for ramping rate of (a) 40 C min−1, (b) 13.3
C min−1, and (c) 3.3
C min−1.
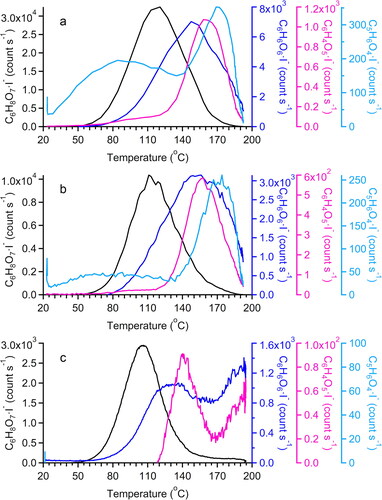
Table 3. Tmax of citric acid (C6H8O7), its primary dehydration product (C6H6O6), secondary dehydration product (C6H4O5), and decarboxylation product of its primary dehydration product (C5H6O4).
