Figures & data
TABLE 1 Description of computational models implemented for droplet evaporation
FIG. 1 Axisymmetric two-dimensional geometry of a spherical droplet in a nearly infinite media (from CitationLongest and Kleinstreuer 2004 with permission).
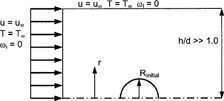
FIG. 2 Representative computational flow field results: (a) Velocity vectors, contours of velocity magnitude, and (b) mass fraction for an axisymmetric 480 cm n-heptane droplet far from the wall with T∞ = 298 K, u∞ = 100 cm/s ω∞ = 0, and Re p = 30.2 (from CitationLongest and Kleinstreuer 2004 with permission).

FIG. 3 Comparison of two-dimensional simulation results for uniform flow to (a) Sherwood and (b) Nusselt number correlations of CitationClift et al. (1978) and CitationRanz and Marshall (1952).
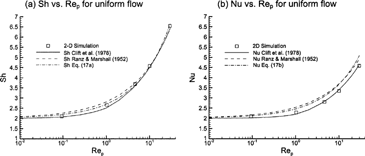
FIG. 4 Computational estimates of normalized droplet surface area (d 2/d o 2) over time compared to the experimental results of CitationRunge et al. (1998) for (a) high volatility n-heptane with T∞ = 298 K and Re p = 30.2; (b) high volatility n-heptane with T∞ = 272 K and Re p = 107.3; (c) low volatility n-decane with T∞ = 272 K and Re p = 94.1; and (d) multicomponent 50:50 heptane-decane mixture with T∞ = 272 K and Re p = 107.0. Descriptions of the numerical models are given in .
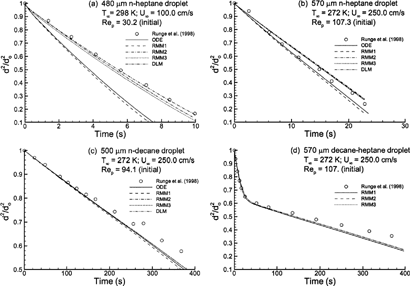
FIG. 5 Variable property (RMM2) estimates of droplet temperature over time for: (a) n-heptane with T∞ = 272 K and Re p = 107.3; and (b) multicomponent 50:50 heptane–decane mixture with T∞ = 272 K and Re p = 107.0.
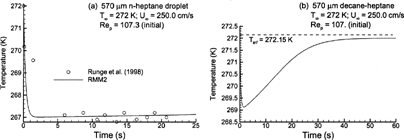
FIG. 6 Semiempirical and resolved-volume simulations of droplet evaporation for a multicomponent 50:50 heptane–decane mixture with T∞ = 298 K and Re p = 107. Results for RMM3 and the DLM are practically indistinguishable from the RMM2 solution.
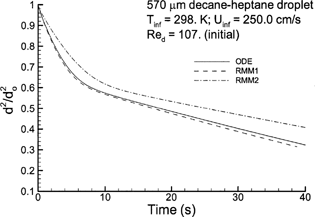
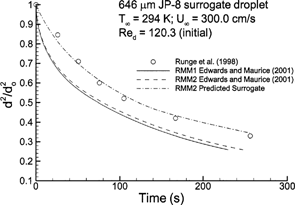
FIG. 7 Computational estimates of normalized droplet surface area (d 2/d o 2 ) over time compared to the experimental results of CitationRunge et al. (1998) for a twelve-component JP-8 surrogate mixture.