Figures & data
Figure 1. The figure shows the model. Only the cost of the prevented relapses is counted in the analysis. Adjuvant trastuzumab is calculated saving 10 or 20% from death of metastatic breast cancer (death 0.8 or 0.9). 1st line therapy (paclitaxel/trastuzumab or docetaxel/trastuzumab) in metastatic setting was calculated administered in the time span 1–6 years following adjuvant chemotherapy (ACT) and 90% (0.9) were concluded candidates for 1st line therapy. 2nd line therapy (vinorelbine or vinorelbine/trastuzumab) was calculated administered in the time span 2–8 years following ACT and two-thirds (0.67) of patients undergoing 1st line therapy was candidates for 2nd line therapy. 3rd line therapy (capecitabine) was calculated administered 4–10 years following ACT and two-thirds (0.67) of patients undergoing 2nd line therapy was candidates for 3rd line therapy. . The figure illustrates the 10 years survival figures published by Bonadonna (CMF regimen) [13], an estimated 5% improved survival due to the FEC regimen [13], the results of the JOINT study [15] and two suggested levels (10% and 20%) of improvement (from the FEC results indicated) due to trastuzumab.
![Figure 1. The figure shows the model. Only the cost of the prevented relapses is counted in the analysis. Adjuvant trastuzumab is calculated saving 10 or 20% from death of metastatic breast cancer (death 0.8 or 0.9). 1st line therapy (paclitaxel/trastuzumab or docetaxel/trastuzumab) in metastatic setting was calculated administered in the time span 1–6 years following adjuvant chemotherapy (ACT) and 90% (0.9) were concluded candidates for 1st line therapy. 2nd line therapy (vinorelbine or vinorelbine/trastuzumab) was calculated administered in the time span 2–8 years following ACT and two-thirds (0.67) of patients undergoing 1st line therapy was candidates for 2nd line therapy. 3rd line therapy (capecitabine) was calculated administered 4–10 years following ACT and two-thirds (0.67) of patients undergoing 2nd line therapy was candidates for 3rd line therapy. Figure 2. The figure illustrates the 10 years survival figures published by Bonadonna (CMF regimen) [13], an estimated 5% improved survival due to the FEC regimen [13], the results of the JOINT study [15] and two suggested levels (10% and 20%) of improvement (from the FEC results indicated) due to trastuzumab.](/cms/asset/823083b7-7b76-40fd-a39a-6f1304a94ccc/ionc_a_209627_f0001_b.gif)
Figure 2. The figure illustrates the 10 years survival figures published by Bonadonna (CMF regimen) [13], an estimated 5% improved survival due to the FEC regimen [13], the results of the JOINT study [15] and two suggested levels (10% and 20%) of improvement (from the FEC results indicated) due to tratuzumab.
![Figure 2. The figure illustrates the 10 years survival figures published by Bonadonna (CMF regimen) [13], an estimated 5% improved survival due to the FEC regimen [13], the results of the JOINT study [15] and two suggested levels (10% and 20%) of improvement (from the FEC results indicated) due to tratuzumab.](/cms/asset/e3da0350-513f-4ee1-bdcc-70c03a3a6121/ionc_a_209627_f0002_b.gif)
Table I. Effectiveness: The table illustrates the calculation of life expectancy following adjuvant chemotherapy containing trastuzumab in early breast cancer.
Table II. The costs per patient treated (in €). Only the costs and savings differing between the two adjuvant regimens (standard FEC100 and FEC100 + Trastuzumab) are included.
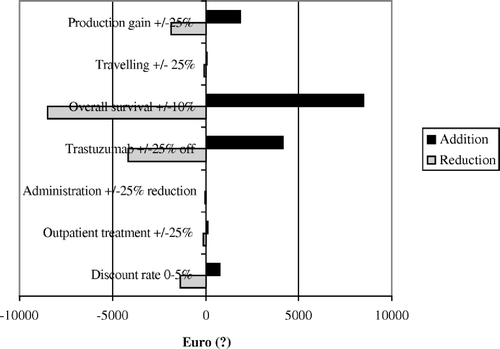
Table III. The table shows cost-effectiveness (C/E) ratios depending on key costing assumptions and 10% or 20% improved overall survival level. Effectiveness was calculated in life years gained (LYG) or QALYs. 3% discount rate was employed.