Figures & data
Table 1. Demographics and clinical characteristics of patients included.
Table 2. Nerve function according to the EORTC CIPN20 questionnaire, mean (SD).
Figure 1. Symptom scores for sensory (A) and motor (B) items in the EORTC QLQ-CIPN20 questionnaire for patients treated with oxaliplatin (black bars) or fluoropyrimidine alone (gray bars). Higher scores mean more symptoms/problems. Data are presented as mean values with standard deviation. ***p < 0.0001; **p < 0.001; *p < 0.05 for comparisons oxaliplatin versus fluoropyrimidine alone groups.
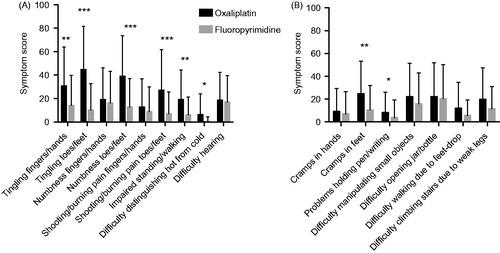
Figure 2. Percent of patients treated with oxaliplatin (black bars) or fluoropyrimidine alone (gray bars) considered to have severe symptoms/problems, i.e. responding ‘Quite a bit’ or ‘Very much’ to the EORTC QLQ-CIPN20 sensory/motor items indicated. ***p < 0.0001; **p < 0.001 for comparisons oxaliplatin versus fluoropyrimidine alone groups.
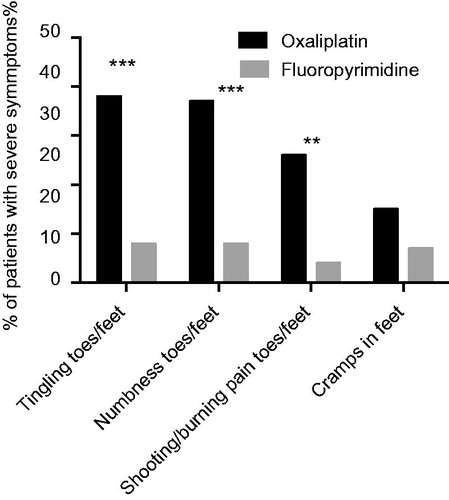
Figure 3. Symptom scores for the EORTC QLQ-CIPN20 sensory/motor items indicated for patients treated with oxaliplatin and divided for gender (A), age (B) and type of regimen (C). Higher scores mean more symptoms/problems. Data are presented as mean values with standard deviation.
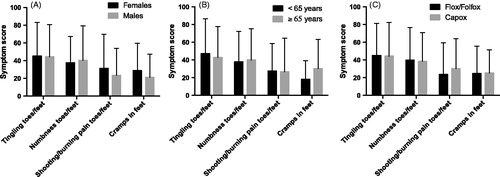
Figure 4. Symptom scores for the EORTC QLQ-CIPN20 items tingling (A), numbness (B), pain (C) and cramps (D) in toes/feet for patients treated with oxaliplatin (black bars) or fluoropyrimidine alone (gray bars) divided into five groups based on time since stop of treatment. Higher score means more symptoms/problems. Data are presented as mean values with standard deviation.
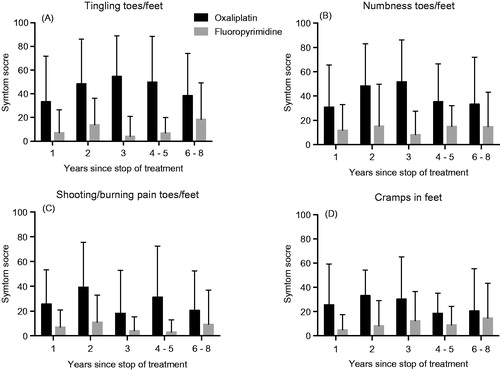
Figure 5. Symptom scores for the EORTC QLQ-CIPN20 items tingling (A), numbness (B), pain (C) and cramps (D) in toes/feet for patients treated with oxaliplatin divided into quartiles for total cumulated dose oxaliplatin received. Higher score means more symptoms/problems. Data are presented as mean values with standard deviation.
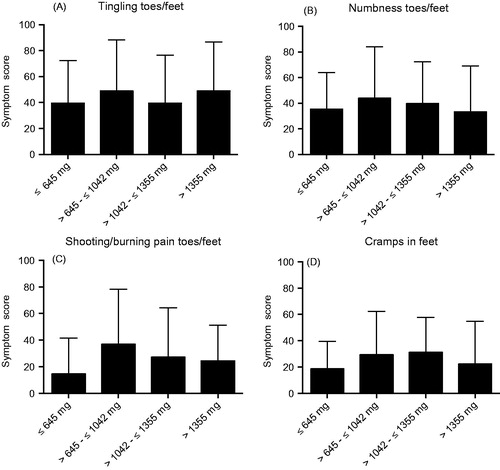
Table 3. Health-related quality of life scores according to the EORTC QLQ-C30, mean (SD).
