Figures & data
Table 1. Distribution of patient and clinicopathological parameters among patients from the BC and DBCG 82b study cohorts.
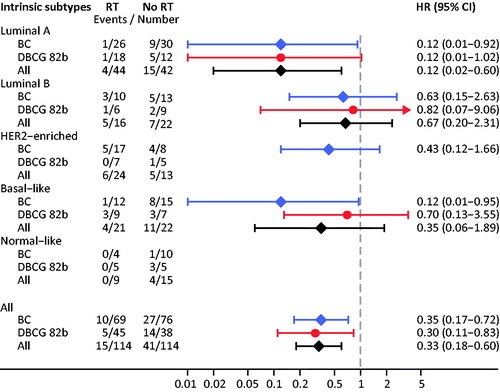
Figure 1. Loco-regional recurrence as a function of randomization assignment to adjuvant postmastectomy radiotherapy (RT) within the study cohorts of the BC-trial (left) and the DBCG 82b-trial (right).
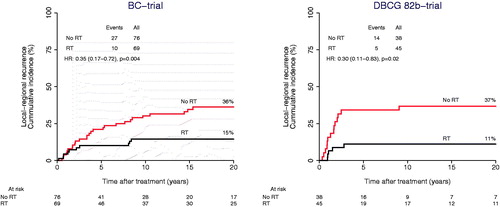
Figure 2. Loco-regional recurrence among patients with a Luminal A tumor as a function of randomization to adjuvant postmastectomy radiotherapy (RT) within the BC-trial (left) and the DBCG 82b-trial (right).
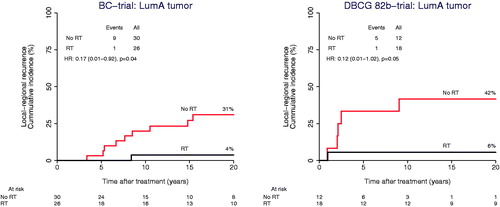
Figure 3. Forest plot showing the association between radiotherapy (RT) and the incidence of local-regional recurrence within different intrinsic subtype subgroups. BC-trial (Blue bar), DBCG 82b-trial (Red bar) and Merged data (Black bar). In subgroups with no events, HR cannot be estimated, nor can the overall HR.