Figures & data
Figure 1. Flow diagram of the inclusion of patients in the present study. Among referred patients, 83% were eligible; a total of 75% filled in the questionnaire at referral. Note: Other reasons for exclusion were referral for genomic profiling for causes other than possible enrollment in a phase 1 cancer trial.
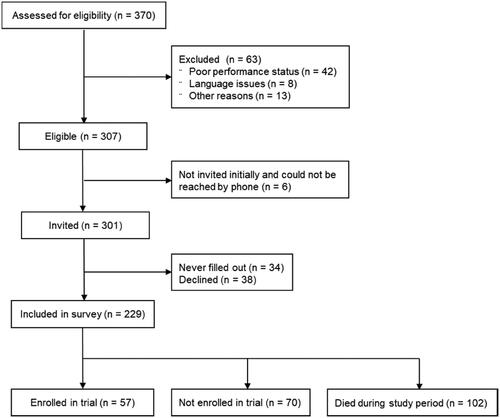
Table 1. Patient characteristics based on self-reported information and patient chart reviews for referred patients (N = 229).
Table 2. Distribution of the severity of stress, anxiety, and depression among patients at first visit (N = 229).
Figure 2. Mean scores of objective understanding of trial information measured with the Quality of Informed Consent Questionnaire, presented for each domain, among enrolled patients (N = 46).
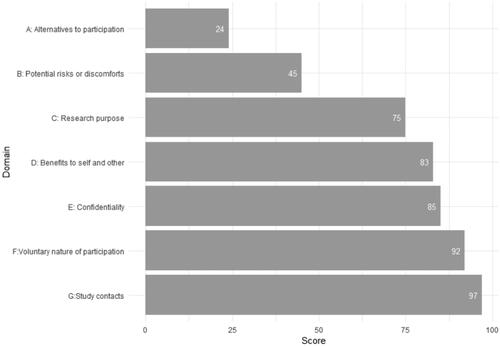
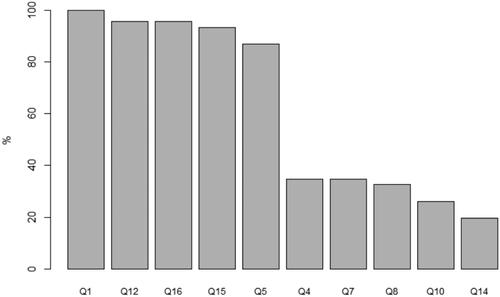
Figure 3. Proportion of patients (N = 46) responding correctly to each statement in the Quality of Informed Consent Questionnaire. Best: Q1. When I signed the consent form for my current cancer therapy, I knew that I was agreeing to participate in a clinical trial (correct answer: agree). Q12. By participating in this clinical trial, I am helping the researchers learn information that may benefit future cancer patients (correct answer: agree). Q16. If I had not wanted to participate in this clinical trial, I could have declined to sign the consent form (correct answer: agree). Q15. The consent form I signed lists the name of the person (or persons) whom I should contact if I have any questions or concerns about the clinical trial (correct answer: agree). Q5. In my clinical trial, one of the researchers’ major purposes is to test the safety of a new drug or treatment (correct answer: agree). Worse: Q4. All the treatments and procedures in my clinical trial are standard for people with my type of cancer (correct answer: disagree). Q7. The treatment being researched in my clinical trial has been proven to be the best treatment for my type of cancer (correct answer: disagree). Q8. In my clinical trial, each group of patients receives a higher dose of the treatment than the group before, until some patients have serious side effects (correct answer: agree). Q10. Compared with standard treatments for my type of cancer, my clinical trial does not carry any additional risks or discomforts (correct answer: disagree). Q14. My doctors did not offer me any alternatives besides treatment in this clinical trial (correct answer: disagree). Q: question.
Data availability statement
Examples of questionnaires, statistical codes, and deidentified participant data are available upon reasonable request.
