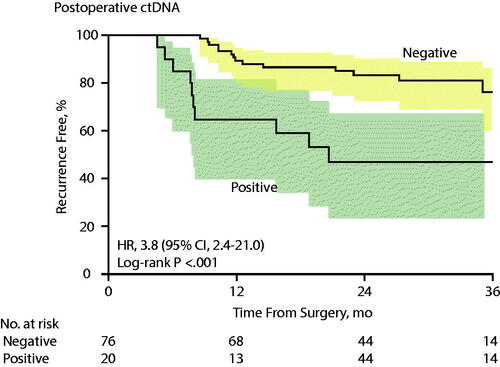
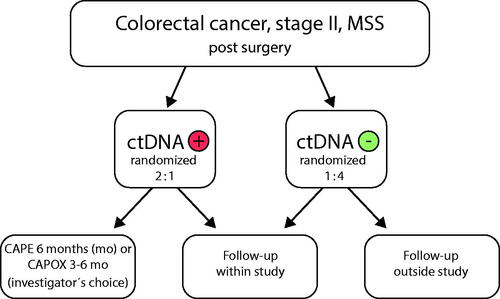
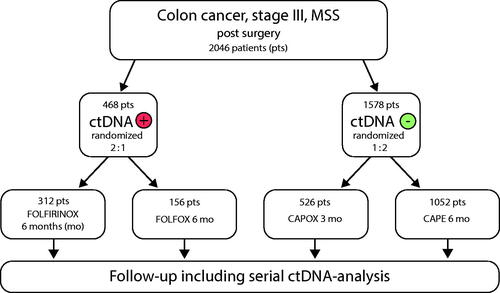
Figures & data
Table 1. Results of trials on ctDNA as a prognostic and predictive marker in early stage colorectal cancer.
Table 2. Ongoing European adjuvant trials in early stage colon cancer evaluating the use of ctDNA.
Table 3. Ongoing non-European adjuvant trials in early stage colon cancer evaluating the use of ctDNA.