Figures & data
Table 1. Demographics and baseline clinical characteristics (adults).
Table 2. Annual exacerbation rates for patients with baseline blood eosinophil counts ≥150 and <150 cells/μL treated with benralizumab plus high-dosage ICS/LABA (adults).
Figure 1. Change from baseline in prebronchodilator FEV1 for patients receiving high-dosage ICS/LABA with baseline eosinophil counts (A, B) ≥ 150 cells/μL and (C, D) < 150 cells/μL for the SIROCCO (A, C) and CALIMA (B, D) studies (adults). FEV1, forced expiratory volume in 1 s; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; Q8W, every 8 weeks (first three doses every 4 weeks). * p < 0.05 for benralizumab Q8W vs. placebo treatment comparison.
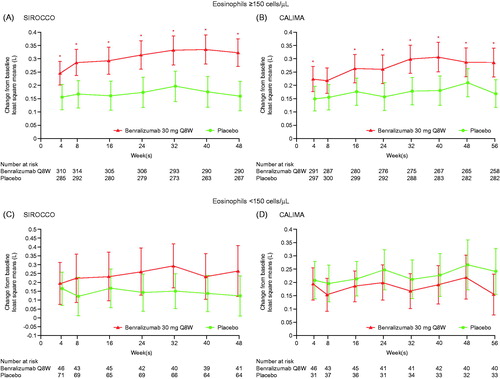
Table 3. Change in key secondary endpoints with benralizumab plus high-dosage ICS/LABA treatment for patients with blood eosinophil counts ≥150 or <150 cells/μL (adults).
Table 4. Change in AQLQ(S) + 12 and ACQ-6 with benralizumab plus high-dosage ICS/LABA treatment for patients with blood eosinophil counts ≥150 or <150 cells/μL (adults).
Table 5. Adverse events, injection-site reactions, and hypersensitivity during on study period (adults).
