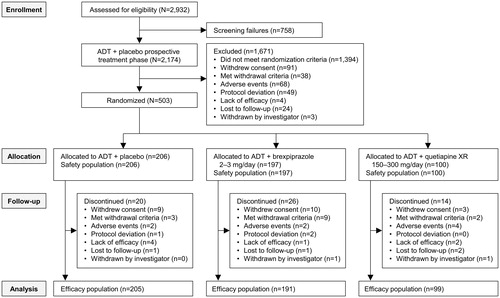
Figures & data
Figure 1. Study design: (A) Blinded study design (as provided in the protocol for the investigators). (B) Unblinded study design (as per IVRS/IWRS design). From the start of prospective treatment, patients visited the study center at weekly intervals for the first 4 weeks and then every 2 weeks for the remainder of the 18-week treatment period. Abbreviations. ADT, antidepressant treatment; CR, controlled-release; IVRS/IWRS, interactive voice/web response system; XR, extended-release.
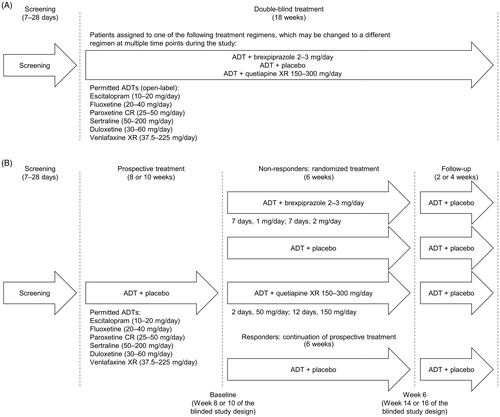
Table 1. Baseline demographic and clinical characteristics, and assigned antidepressant treatment (randomized population).
Figure 3. Effects of brexpiprazole, placebo, and quetiapine XR as adjunct to antidepressant treatment on Montgomery–Åsberg Depression Rating Scale (MADRS) total score (efficacy population). *p < .05, **p < .01, ***p < .001 vs placebo. Baseline MADRS total scores: ADT + placebo = 25.4; ADT + brexpiprazole = 25.3; ADT + quetiapine XR = 25.6. Abbreviations. ADT, antidepressant treatment; LS, least squares; SE, standard error; XR, extended-release.
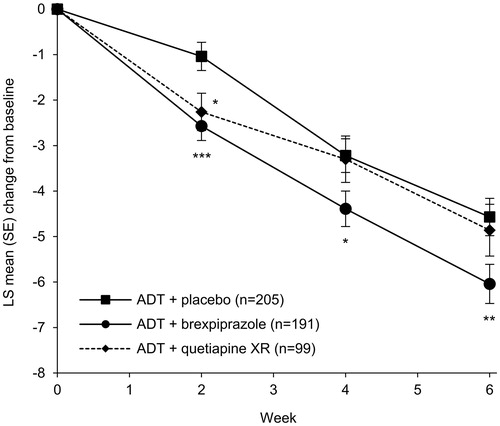
Table 2. Effects of brexpiprazole, placebo, and quetiapine XR as adjunct to antidepressant treatment on efficacy endpoints at week 6 (efficacy population).
Figure 4. Effects of brexpiprazole, placebo, and quetiapine XR as adjunct to antidepressant treatment on Sheehan Disability Scale (SDS) scores at week 6 (efficacy population). *p < .05 vs placebo, in favor of active treatment (considered nominal within the formal testing hierarchy). Baseline SDS mean scores: ADT + placebo = 5.7; ADT + brexpiprazole = 5.6; ADT + quetiapine XR = 5.6. Patient numbers for the work/studies item: ADT + placebo, n = 136; ADT + brexpiprazole, n = 125; ADT + quetiapine XR, n = 67. Abbreviations. ADT, antidepressant treatment; LS, least squares; SE, standard error; XR, extended-release.
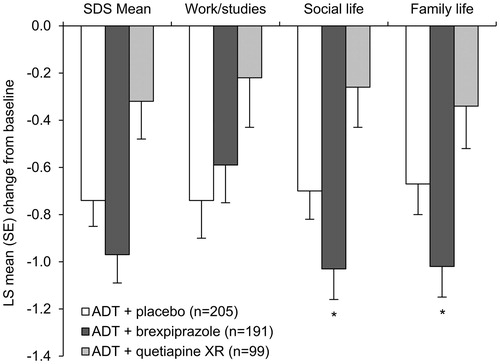
Table 3. Treatment-emergent adverse events (TEAEs) from baseline of the randomized treatment phase (safety population).
Table 4. Laboratory assessments (safety population).