Figures & data
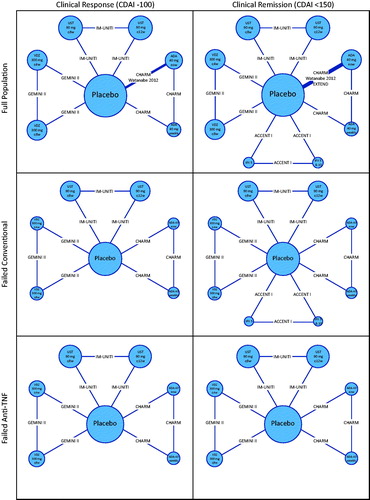
Figure 2. Placebo response/remission rates for clinical response and clinical remission for maintenance phase trialsCitation7,Citation25–27,Citation31,Citation32. Abbreviations. ADA, Adalimumab; CDAI, Crohn’s Disease Activity Index; IFX, Infliximab; NA, Not available; VDZ, Vedolizumab; UST, Ustekinumab.
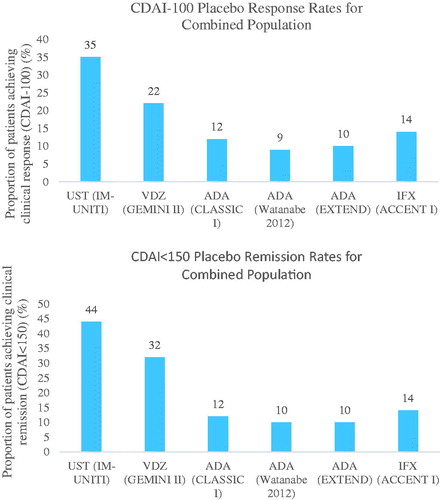
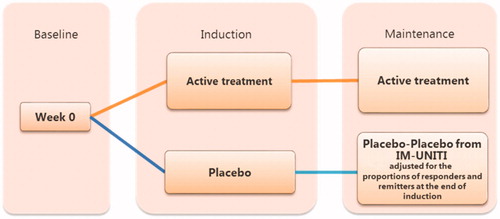
Figure 4. Treatment sequence analysis strategy. Abbreviations. CDAI, Crohn’s Disease Activity Index; UST, Ustekinumab.
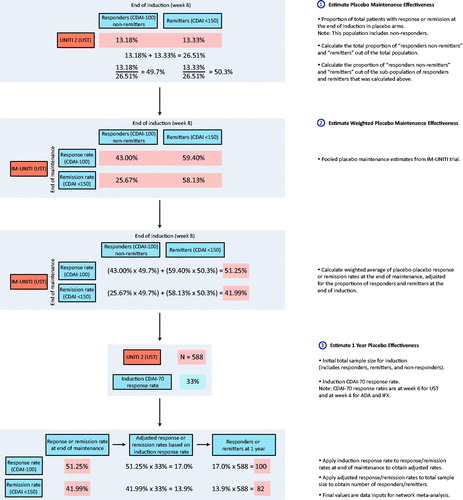
Table 1. Treatment sequence calculations for the overall population.
Table 2. Results from treatment sequence network meta-analysis for the overall population, failed conventional and failed anti-TNF-α sub-populations.
Table 3. Results from sensitivity analyses.
Appendix 1. Search strategy.MEDLINE and MEDLINE-IN-PROCESS (date of the search: 2 July 2015)
EMBASE (date of the search: 2 July 2015)
The Cochrane Library (date of the search: 2 July 2015)
Appendix 2. Treatment doses in moderate-to-severe Crohn’s disease.
Appendix 3. Detailed rationale for included and excluded studies.Included studies for primary dosage network meta-analysis
Included studies for failed conventional sub-population primary dosage network meta-analyses
Included studies for the failed anti-TNF sub-population primary dosage network meta-analyses
Excluded studies from primary dosage network meta-analysis
Appendix 4. Quality assessment of included studies.
Appendix 5. ARisk of bias assessment.
Appendix 6. Study design of maintenance phase trials.
Appendix 7. Placebo response/remission rates versus treatment response/remission rates and odds ratios.
Appendix 8. Detailed summary of randomized controlled trial characteristics.Induction
Maintenance
Appendix 9. Treatment sequence calculations for sub-populations.
Treatment sequence calculations for failed anti-TNF sub-population
Appendix 10. Results from network meta-analysis based on data prior to treatment sequencing adjustments.
Appendix 11. Random effects model results.Results from primary analyses for random effects model
Appendix 13. PRISMA network meta-analysis checklist.