Figures & data
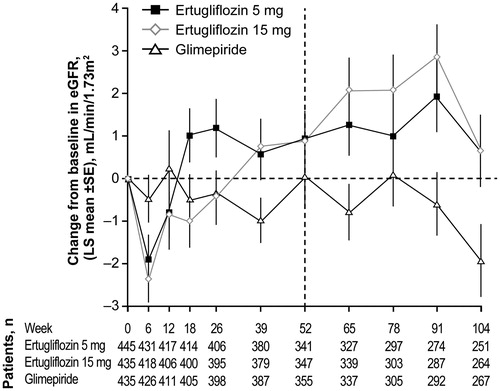
Figure 1. Design of the VERTIS SU study. X-axis values are weeks. aPatients on one of the following regimens were also eligible to enter the screening period, and could enroll in the study if they met entry criteria after the wash-off/dose titration/stabilization period: on metformin monotherapy ≥1500 mg/day <8 weeks, HbA1c ≥7.0% and ≤9.0% (≥53 mmol/mol and ≤75 mmol/mol) – patients were to maintain metformin dose ≥1500 mg/day for ≥8 weeks; on metformin monotherapy <1500 mg/day and with HbA1c ≥7.5% and ≤9.5% (≥58 mmol/mol and ≤80 mmol/mol) – patients were titrated to metformin ≥1500 mg/day and were to maintain metformin dose for ≥8 weeks; on metformin + single allowable antihyperglycemic agentd and HbA1c ≥6.5% and ≤8.5% (≥48 mmol/mol and ≤69 mmol/mol) – patients were to discontinue non-metformin AHA, titrate metformin to ≥1500 mg/day (if necessary), and maintain metformin dose ≥1500 mg/day for ≥8 weeks (≥10 weeks for patients discontinuing SU therapy). bGlimepiride was initiated at 1 mg once daily and up-titrated to a maximum of 6 or 8 mg/day according to the local label or maximum tolerated dose. cPatients rescued with sitagliptin in Phase A were not eligible to enter Phase B; patients were not rescued during Phase B. dThis included SUs at <50% of the maximum approved dose in the local country label, dipeptidyl peptidase-4 inhibitors, meglitinides or alpha glucosidase inhibitors. Abbreviations. AHA, Antihyperglycemic agent; HbA1c, Glycated hemoglobin; SU, Sulfonylurea; R, Randomization.
Table 1. Demographics and baseline disease characteristics.
Figure 2. Change from baseline over time in (A) HbA1c (FAS), (B) HbA1c (per protocol analysis), (C) body weight (FAS) and (D) SBP (FAS), through Week 104. Vertical dashed line indicates Week 52 (primary time point). Abbreviations. BL, Baseline; FAS, Full analysis set; HbA1c, Glycated hemoglobin; LS, Least squares; SBP, Systolic blood pressure; SE, Standard error.
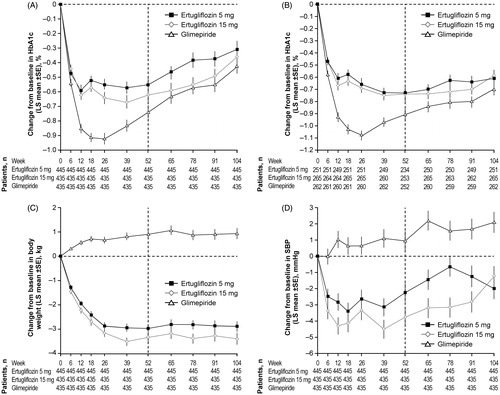
Table 2. Summary of glycemic-related endpoints.
Table 3. Summary of other efficacy endpoints at Week 104 (full analysis set).
Figure 3. Proportion of patients meeting composite endpoint at Week 104 of (A) HbA1c reduction >0.5% (5.5 mmol/mol) with no symptomatic hypoglycemia or body weight gain and (B) HbA1c <7.0% (53 mmol/mol) with no symptomatic hypoglycemia. Abbreviation. HbA1c, Glycated hemoglobin.
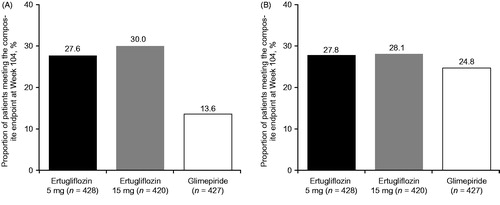
Table 4. Summary of overall safety and prespecified AEs.
Supplemental Materials
Download MS Word (210.7 KB)Data sharing
MSD’s data sharing policy, including restrictions, is available at http://engagezone.merck.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to [email protected].