Figures & data
Figure 1. Patient disposition. Abbreviation. CCP+, cyclic citrullinated peptide positive. This figure was originally published on poster 2500, “Association Between Anti-Citrullinated Protein Antibody Status, Erosive Disease and Healthcare Resource Utilization in Patients With RA”; Leslie R. Harrold”, presented at the 2018 Annual Meeting of the American College of Rheumatology held in Chicago, IL, USA.
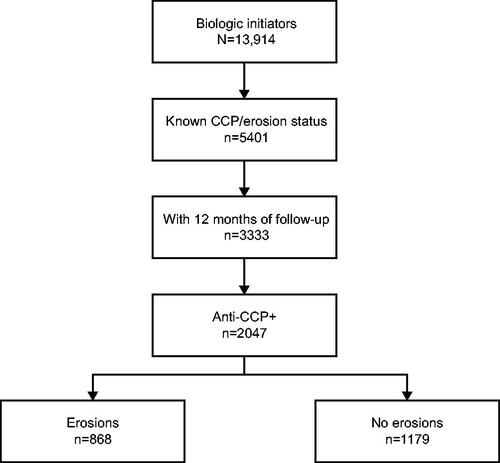
Table 1. Patient demographics and disease characteristics at first biologic initiation visit.
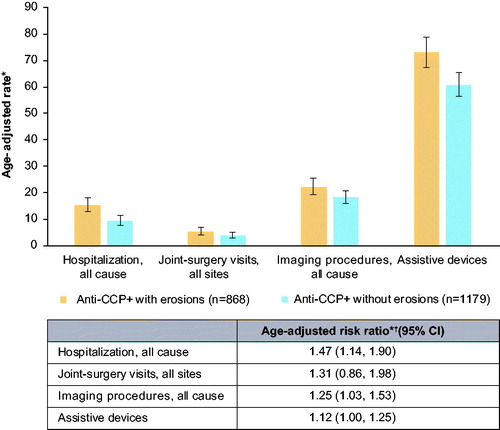
Figure 2. Rates of HCRU in anti-CCP + patients with RA, with and without erosions. *Rates per 100 patient-years with 95% CI based on Poisson distributed counts. †Reference group: anti-CCP + patients without erosions. Abbreviations. CCP+, cyclic citrullinated peptide positive; CI, confidence interval; HCRU, healthcare resource utilization; RA, rheumatoid arthritis.
Data availability statement
Bristol-Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The Corrona dataset is based on a large US multicenter study adhering to a number of institutional review boards, with complex logistics. Patients did not provide consent to raw data sharing during the data collection for this purpose, and the Corrona data sharing policies do not permit raw data sharing for this purpose. An aggregated limited dataset from the current analyses is available to qualified investigators with an approved protocol. Data requests may be sent to Corrona, represented by Dr. Jeff Greenberg, e-mail [email protected].
