Figures & data
Table 1. Study designs and interventions investigated in ATTR-PN trials.
Table 2. Summary of key eligibility criteria in ATTR-PN trials.
Figure 1. Evidence networks in ATTR-PN trials. (A) Network of RCTs for the Norfolk TQOL and safety outcomes (any adverse events, serious adverse events, and withdrawals due to adverse events). (B) Network of RCTs for the mBMI outcome. Abbreviations. INO, Inotersen; mBMI, Modified body mass index; PAT, Patisiran; PBO, Placebo; RCT, Randomized controlled trial; TAF, Tafamidis; TQOL, Total Quality of Life Score.
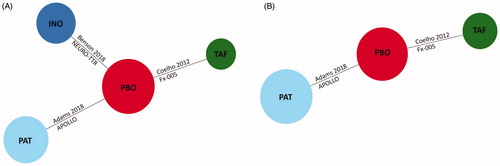
Figure 2. Distribution of baseline disease-related characteristics across ATTR-PN trials. Abbreviations. ATTR-PN, Transthyretin amyloidosis with polyneuropathy; TTR, Transthyretin; Val30Met, Valine to methionine point mutation at position 30.
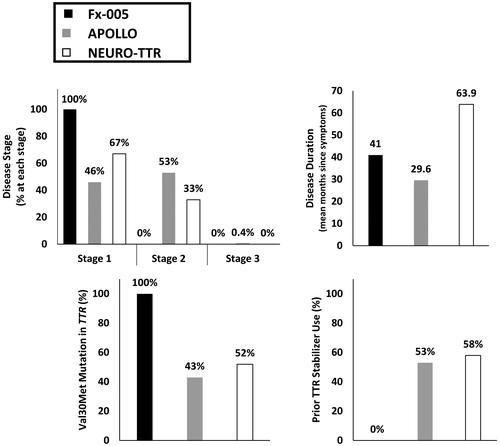
Figure 3. Distribution of baseline demographic characteristics across ATTR-PN trials. Abbreviations. ATTR-PN, Transthyretin amyloidosis with polyneuropathy; Eur., Europe; N. Am., North America; S. Am., South America; W. Eur., Western Europe.
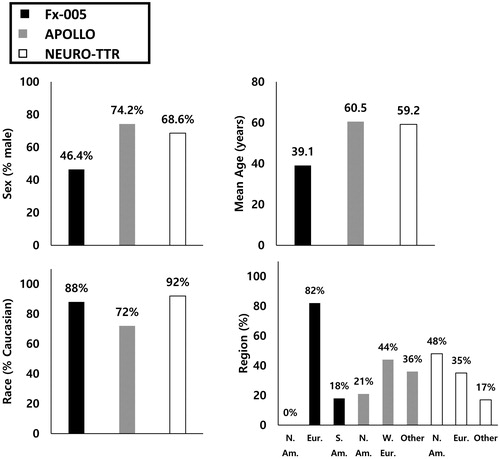
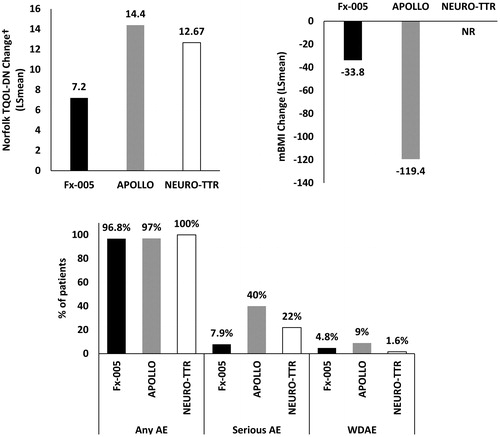
Figure 4. Placebo response for commonly defined outcomes across ATTR-PN trials (ITT populations). †Small numerical difference in range reported for the Norfolk TQOL scale between studies. Abbreviations. AE, Adverse event; ATTR-PN, Transthyretin amyloidosis with polyneuropathy; ITT, Intention to treat; LS, Least squares; mBMI, Modified body mass index; NR, Not reported; SAE, Serious adverse event; TQOL, Total Quality of Life; WDAE, Withdrawal due to adverse events.