Figures & data
Figure 1. Concentration-dependent functional effects of a partial nAChR agonist. Short exposures to high concentrations (μM) of a partial agonist cause activation of nAChRs (dotted line). A partial agonist like varenicline causes only partial activation (here 20%) versus the full agonist ACh that causes maximal nAChR activation (100%). Prolonged exposures to low partial agonist concentrations (nM) causes receptor desensitization, shown by the decrease in the ACh response in the presence of increasing concentrations of the partial agonist (solid line). In the sustained presence of low agonist concentrations present in human brain (nM range, gray bar), only a small fraction of the nAChRs that are not desensitized can be activated, resulting in extensive nAChR desensitization and low-level activation at steady state. Abbreviations. Ach, acetylcholine; nAChRs, nicotinic acetylcholine receptors.
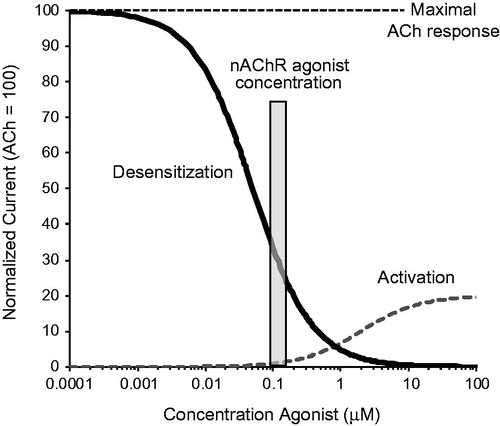
Figure 2. Comparison of agonist and antagonist activities of NRT, cytisine and varenicline. (A) The degree of activation and desensitization (agonist activity) of α4β2 and α6β2* nAChRs by clinical doses of each agonist relative to the effects of nicotine from smoking (= 100%). (B) The decrease in nicotine receptor occupancy of α4β2 and α6β2* nAChRs (antagonist activity) by clinical doses of each agonist relative to the decrease in nicotine receptor occupancy when smoking without nAChR agonist treatment (= 0%). Bars represent the magnitude of activities expressed as a percentage of the corresponding effects of nicotine from smoking. Smoking activation and desensitization = 100%, decrease in nicotine receptor occupancy = 0%, indicated by dotted lines. Abbreviations. nAChRs, nicotinic acetylcholine receptors; NRT, nicotine replacement therapy; RO, receptor occupancy.
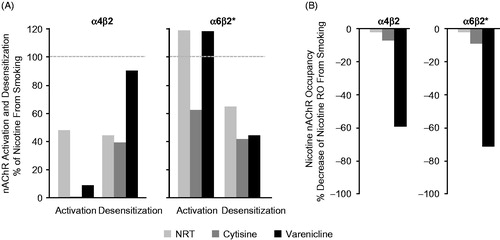
Figure 3. Comparison of continuous abstinence rates in randomized trials that were included in the clinical program: 12 weeks’ varenicline treatment for smoking cessation at weeks 9‒12. Pfizer-sponsored studies by (A) General populations, (B) Comorbid populations or (C) Alternative treatment paradigms. *Varenicline 1 mg BID vs. placebo or active treatment comparator: difference significant at week 12; N, intention-to-treat population (varenicline plus placebo or active treatment comparator). ORs (95% CI) are varenicline vs. placebo or active treatment comparator. Abbreviations. BID, twice daily; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; NPS, neuropsychiatric; NRT, nicotine replacement therapy; OR, odds ratio.
Table 1. Extended use and other alternative treatment paradigms.
Table 2. Neuropsychiatric adverse events reported in the EAGLES randomized controlled safety trial: incidence of the primary composite endpoint and selected components.
Table 3. Meta-analyses of randomized controlled trials examining neuropsychiatric adverse events related to varenicline.
