Figures & data
Table 1. Phase III VERTIS clinical studies included in the pooled analyses.
Table 2. Baseline demographics and disease characteristics of patients included in the pooled efficacy (placebo pool) and safety analyses (broad pool).
Figure 1. Least squares mean change from baseline at Week 26 in (A) HbA1c, (B) body weight and (C) SBP by racial subgroup (placebo pool).
aPlacebo-adjusted difference in LS means (95% CI).
Abbreviations. HbA1c, Glycated hemoglobin; LS, Least squares; SBP, Systolic blood pressure.
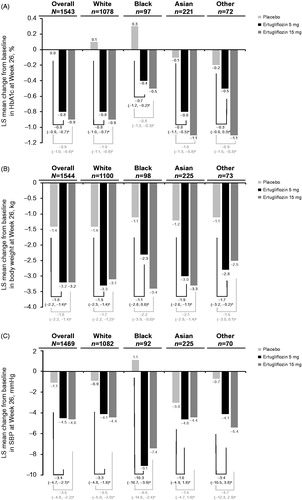
Table 3. Summary of AEs and pre-specified AEs.
Data availability statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information) Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
