Figures & data
Table 1. LCSS scores: baseline and change from baseline – difference between treatment arms.
Figure 1. Time to deterioration (TtD) in LCSS. A forest plot is shown to display the TtD hazard ratios for the LCSS data for the six symptom-specific items and three summary items, as well as the Total Score (mean of the nine LCSS items) and the Average Symptom Burden Index (ASBI; mean of the 6 symptom items). Abbreviations. HR, Hazard ratio; PboErl, Placebo + erlotinib treatment arm; RamErl, Ramucirumab + erlotinib treatment arm.
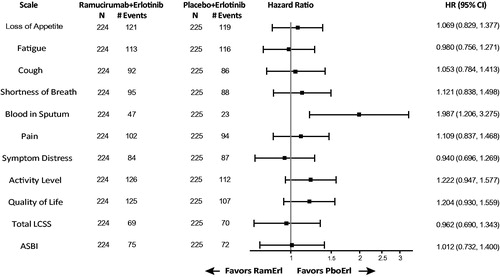
Figure 2. Kaplan–Meier estimates of time to deterioration for (A) Total LCSS and (B) ASBI. Abbreviations. HR, Hazard ratio; NR, Not reached; PboErl, Placebo + erlotinib treatment arm; RamErl, Ramucirumab + erlotinib treatment arm.
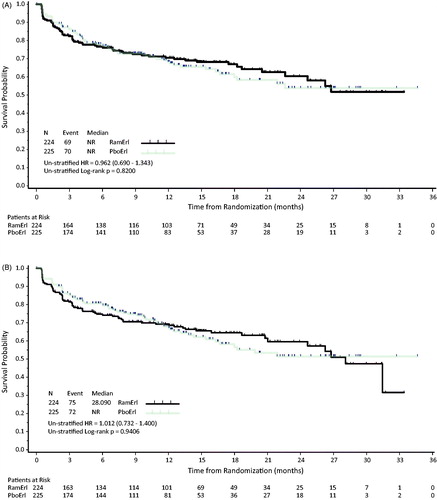
Figure 3. LCSS Total Score by cycle. For the 100 point LCSS scale, the mean LCSS Total Score ± SD is shown for each cycle for the two treatment arms. Higher mean LCSS scores indicate worse symptom burden. Abbreviations. PboErl, Placebo + erlotinib treatment arm; RamErl, Ramucirumab + erlotinib treatment arm.
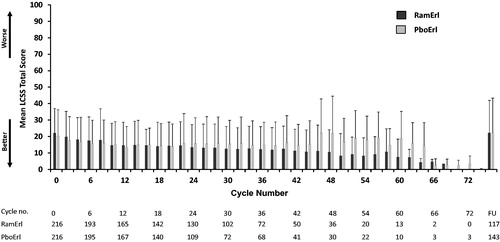
Figure 4. Mean EQ-5D Index Scores (±SD) across treatment cycles. Patients completed the EQ-5D at baseline (Cycle/Visit 0) and every other subsequent visit. The mean EQ-5D Index score for each treatment arm is shown over time, including the 30 day post-discontinuation follow-up visit. Abbreviations. PboErl, Placebo + erlotinib treatment arm; RamErl, Ramucirumab + erlotinib treatment arm.
Data availability statement
Eli Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the Vivli website: www.vivli.org.
