Figures & data
Table 1. Detailed criteria for inclusion/exclusion of studies for epidemiology SLR.
Table 2. Detailed criteria for inclusion/exclusion of studies for clinical burden SLR.
Figure 1. PRISMA diagram: Epidemiology. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; y/o, years old.
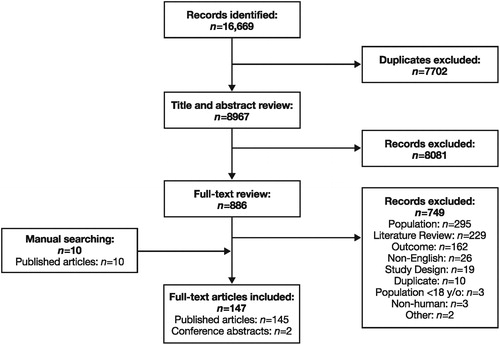
Table 3. Most commonly reported comorbidities for patients with CRSwNPa.
Table 4. Drugs most commonly used for CRSwNPa.
Figure 2. PRISMA diagram for RCTs: Clinical Burden. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCTs, randomized clinical trials.
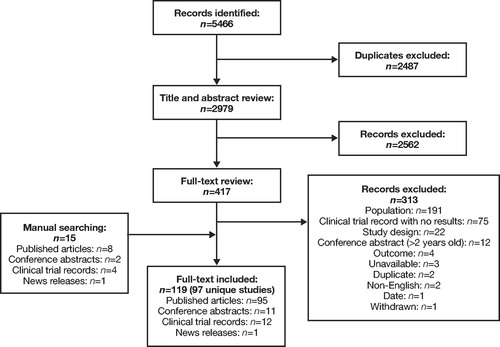
Figure 3. PRISMA diagram for RWE studies: Clinical Burden. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RWE, real-world evidence.
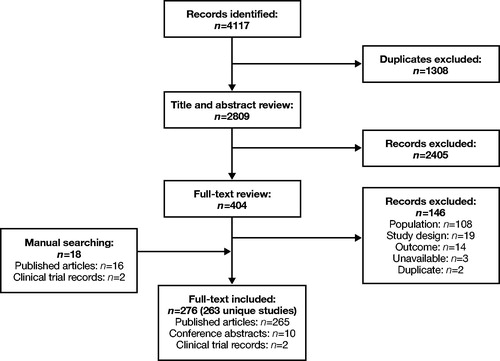
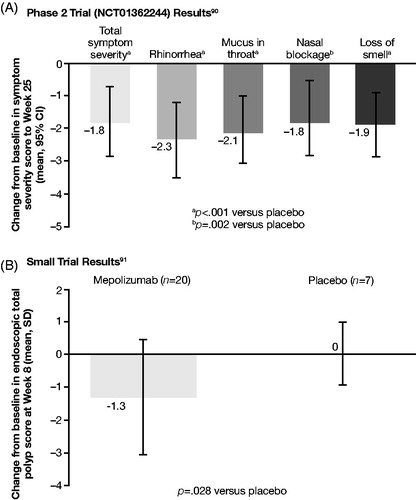
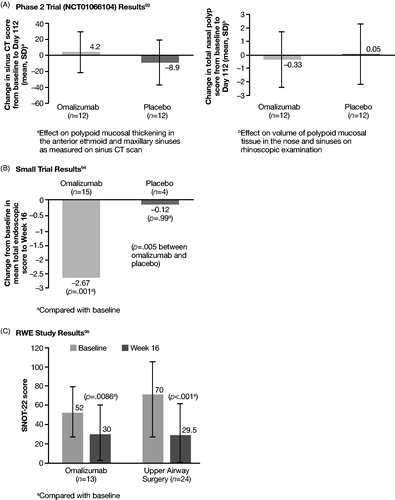
Table 5. RCTs and RWE studies investigating biologicsa.
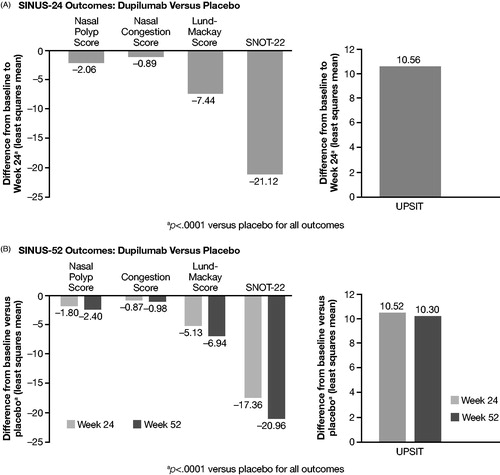
Figure 4. Clinical outcomes scores for dupilumab versus placebo from SINUS-24 and SINUS-52 Phase III RCTs.