Figures & data
Table 1. Demographics and baseline characteristics.
Table 2. Rates and risk (time-to-first analysis) of on-treatment moderate/severe exacerbation.
Figure 1. Time-to-first on-treatment moderate/severe exacerbation in (A) China; (B) overall ITT*. Abbreviations. FF, fluticasone furoate; ITT, intent-to-treat; UMEC, umeclidinium; VI, vilanterol. *From Lipson et al.Citation10. Copyright © 2018 Massachusetts Medical Society, Reprinted with permission from Massachusetts Medical Society.
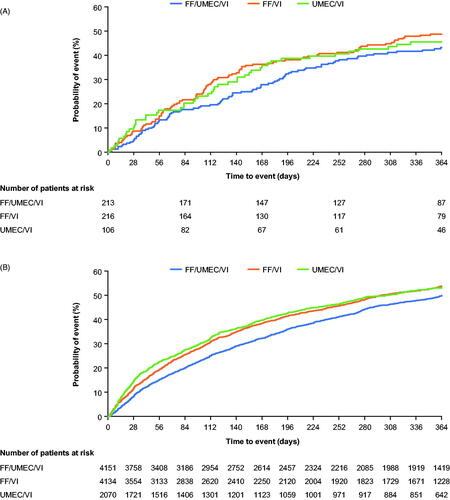
Figure 2. Change from baseline in trough FEV1. Abbreviations. CI, confidence interval; FEV1, forced expiration volume in 1 second; FF, fluticasone furoate; ITT, intent-to-treat; LS, least squares; UMEC, umeclidinium; VI, vilanterol.
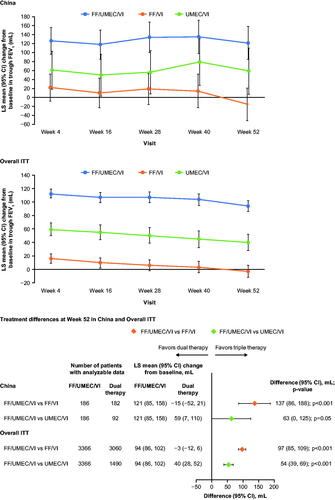
Figure 3. Odds of SGRQ response with FF/UMEC/VI versus dual therapy comparators at Week 52. Response is defined as a decrease from baseline in SGRQ total score of ≥4 units. Non-response is defined as a decrease from baseline in SGRQ total score <4 units below baseline, or an increase from baseline in SGRQ total score or a missing SGRQ total score with no subsequent on-treatment scores. Patients did not have a responder status derived if baseline SGRQ total score was missing, or if the SGRQ total score at a particular visit was missing but subsequent on-treatment SGRQ total scores were present. Abbreviations. n, number of responders; N, total number of analyzable patients; CI, confidence interval; FF, fluticasone furoate; ITT, intent-to-treat; SGRQ, St George’s Respiratory Questionnaire; UMEC, umeclidinium; VI, vilanterol.
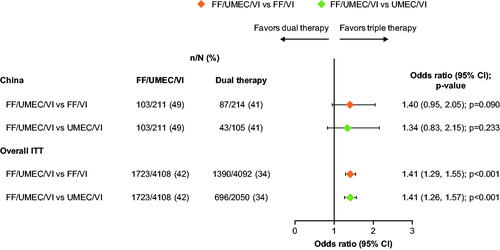
Figure 4. Odds of CAT response with FF/UMEC/VI versus dual therapy comparators at Week 52. Response is defined as a decrease from baseline in CAT score of ≥2 units. Non-response is defined as a decrease from baseline in CAT score <2 units, or an increase from baseline in CAT score, or a missing CAT score with no subsequent non-missing on-treatment scores. Patients did not have a responder status derived if baseline CAT score was missing but subsequent on-treatment CAT scores were present. Abbreviations. CAT; COPD Assessment Test; n, number of responders; N, total number of analyzable patients; CI, confidence interval; FF, fluticasone furoate; ITT, intent-to-treat; UMEC, umeclidinium; VI, vilanterol.
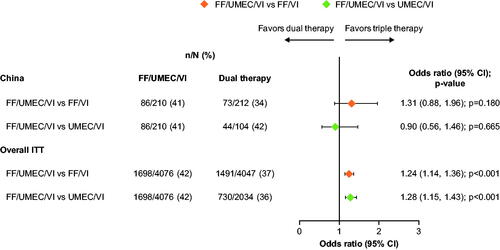
Table 3. Summary of on-treatment AESI and adjudicated SAEs.
Supplemental Material
Download MS Word (294.5 KB)Data availability statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
