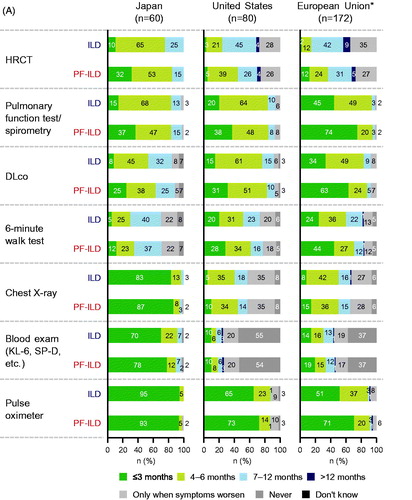
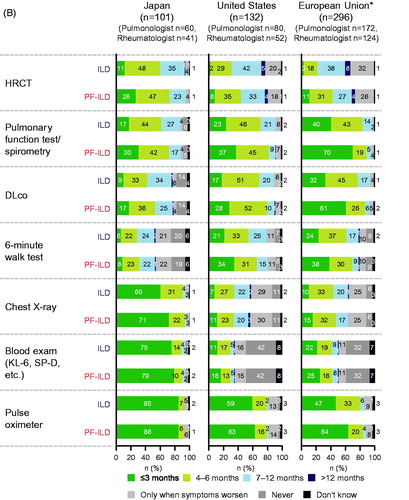
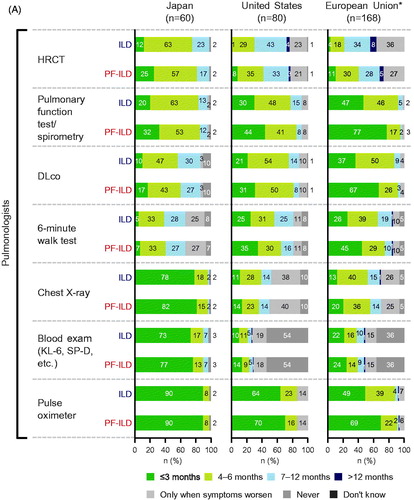
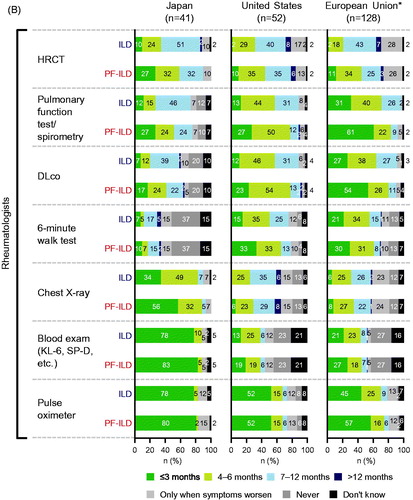
Figures & data
Table 1. Characteristics of physicians who responded to the survey.
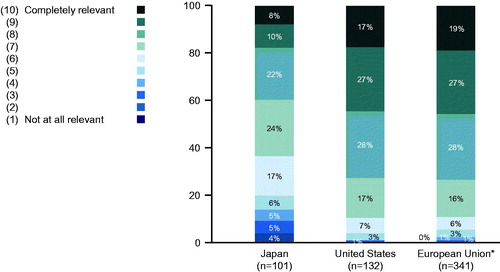
Figure 1. Relevance of the progressive fibrosing phenotype of interstitial lung disease (ILD) by region (on a scale from 1–10, where 1 is “not at all relevant” and 10 is “completely relevant”). *Germany, France, United Kingdom, Italy, and Spain. The following questions were asked in this portion of the survey: For the following course of the interview please carefully read the following definition. In this research, progressive fibrosing ILD is defined as a phenotype that might occur in different forms of ILD, characterized by: (1) progression in terms of deterioration of lung function (FVC = forced vital capacity) and/or worsening of respiratory symptoms, and/or increasing extent of fibrotic changes on chest images despite treatment with (unapproved) medication used in clinical practice to treat ILD AND (2) the presence of fibrosis detected by high resolution CT (i.e. reticular abnormality with traction bronchiectasis, with or without honeycombing) The terminology to describe this phenotype is progressive fibrosing ILD (PF-ILD). How relevant is this definition—patients with a common progressive fibrosing ILD clinical behavior irrespective of different forms of ILD—for you?
Supplemental Material
Download PDF (97.6 KB)Data availability statement
To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to all relevant material, including participant-level clinical study data, and relevant material as needed by them to fulfill their role and obligations as authors under the ICMJE criteria. Furthermore, clinical study documents (e.g. study report, study protocol, statistical analysis plan) and participant clinical study data are available to be shared after publication of the primary manuscript in a peer-reviewed journal and if regulatory activities are complete and other criteria met per the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data: https://trials.boehringer-ingelheim.com/. Prior to providing access, documents will be examined, and, if necessary, redacted and the data will be de-identified, to protect the personal data of study participants and personnel, and to respect the boundaries of the informed consent of the study participants. Clinical Study Reports and Related Clinical Documents can also be requested via the link https://trials.boehringer-ingelheim.com/. All requests will be governed by a Document Sharing Agreement. Bona fide, qualified scientific and medical researchers may request access to de-identified, analyzable participant clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a Data Sharing Agreement, data are shared in a secured data-access system for a limited period of 1 year, which may be extended upon request. Researchers should use the https://trials.boehringer-ingelheim.com/ link to request access to study data.