Figures & data
Figure 1. Flow chart of the exploratory phase, consensus process, and patient focus group analysis. Timeline of the methods for development of the EndoWheel tool. aIndividuals consulted included a pain specialist, a fertility specialist, general practitioners, community physicians, patient advocates, and patients. bThe Delphi process was used, with voting on a 9-point scale. Consensus was defined as both ≥80% of respondents indicating high agreement scores in the range of 7–9 and ≤20% indicating low agreement scores in the range of 1–3.
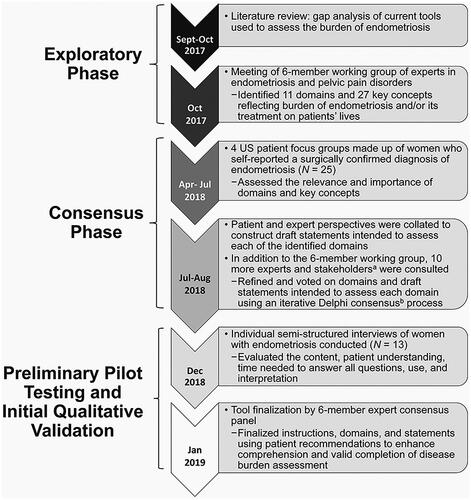
Figure 2. Questionnaire and image of the assessment EndoWheel tool. The tool and the 12 impacts of endometriosis are assessed. Patients are instructed to circle numeric rating scale scores based on their responses to each statement.
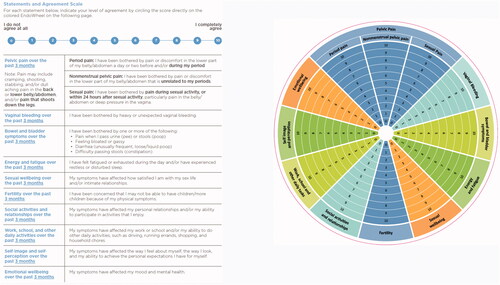
Figure 3. Examples of possible completed EndoWheel assessments. The size of the area formed from connecting the scores in each domain allows for visualization of the disease burden.
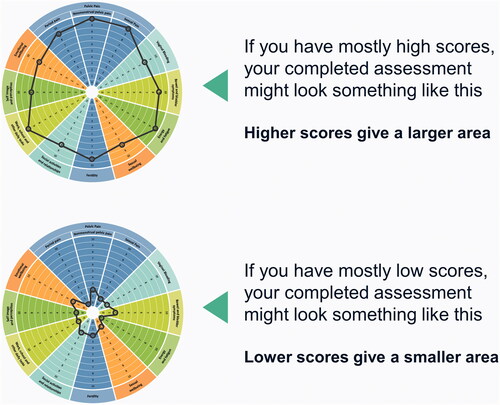
Table 1. Baseline demographics and characteristics of consensus-phase focus group participants.
Table 2. Baseline demographics and characteristics of preliminary pilot testing and initial qualitative analysis of individual interviewees.
Data availability statement
AbbVie is committed to responsible data sharing regarding the studies we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g. protocols and Clinical Study Reports), if the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This data set can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.AbbVie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
