Figures & data
Figure 1. The medicine adoption model. Abbreviations. PH–: non-pharmaceutical company stakeholder groups; PH+: pharmaceutical company stakeholder groups.
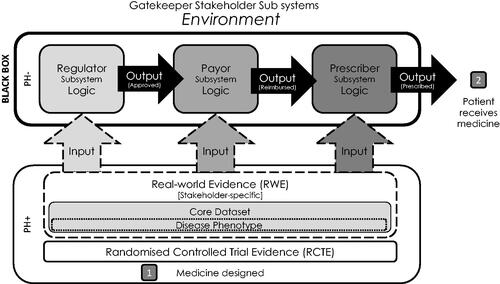
Figure 2. Expected beneficial effect on time to maximal adoption (tmAd) of a new medicine when arriving at its launch with phase III and real-world evidence. Abbreviations. Comm, Commercial; MAcc, Market Access; MAff, Medical Affairs; Raff, Regulatory Affairs; Payo, Payors; Pres, Prescribers.
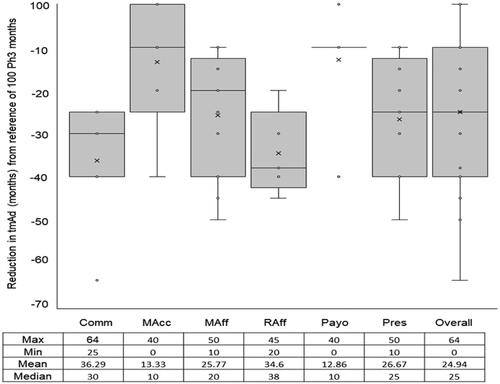
Figure 3. Expected beneficial effect on depth of maximal adoption (dmAd) of a new medicine when arriving at its launch with phase III and real-world evidence. Abbreviations. Comm, Commercial; MAcc, Market Access; MAff, Medical Affairs; Raff, Regulatory Affairs; Payo, Payors; Pres, Prescribers.
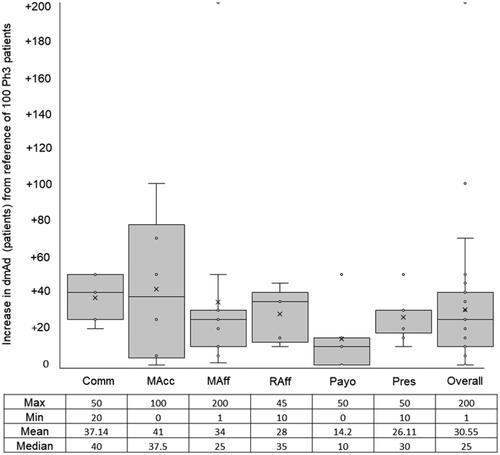
Figure 4. Expected beneficial effect on time to maximal adoption (tmAd) and depth of maximal adoption (dmAd) of a new medicine when arriving at its launch with phase III and real-world evidence. Abbreviaitons. Comm, Commercial; MAcc, Market Access; MAff, Medical Affairs; Raff, Regulatory Affairs; Payo, Payors; Pres, Prescribers.
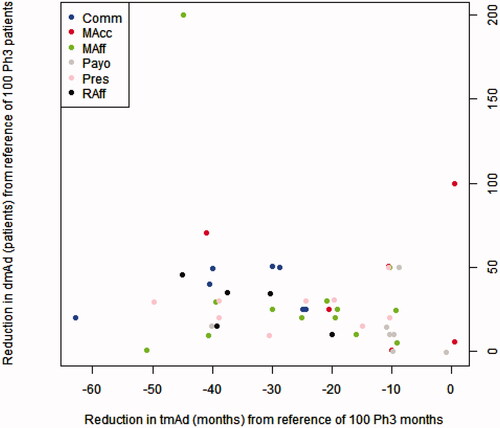
Table 1. Analysis of SRs above the consensus threshold for inclusion (CI > 0.50) in ≥ 4 SHGs and their heterogeneity of consensus scores regarding the time to maximal adoption (tmAd).
Table 2. Analysis of SRs above the consensus threshold for inclusion (CI > 0.50) in ≥ 4SHGs and their heterogeneity of consensus scores regarding the depth of maximal adoption (dmAd).
Table 3. Number of Unique Supporting Rationales (SRs) suggested by SHGs as a proportion of the total number of SRs suggested regarding time to maximal adoption (tmAd) and depth of maximal adoption (dmAd).
Table 4. Supporting Rationales (SRs) for expected effect sizes by subsystem, commonality and mode of effect: via input or logic.
Data availability statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
