Figures & data
Table 1. Differences in baseline characteristics.
Table 2. Response rates and comparative efficacy for cilta-cel versus ide-cel.
Table 3. Estimated medians and comparative efficacy for cilta-cel versus ide-cel.
Figure 1. Observed (unadjusted) and adjusted (base case) Kaplan–Meier plots of a duration of response. Note. Base case results adjusted for refractory status, cytogenetic profile, revised International Staging System stage, and all plasmacytomas. Abbreviations: ESS, effective sample size.
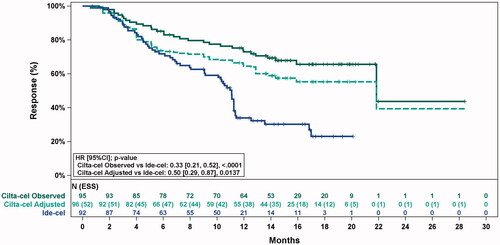
Figure 2. Observed (unadjusted) and adjusted (base case) Kaplan–Meier plots of progression-free survival. Note. Base case results adjusted for refractory status, cytogenetic profile, revised International Staging System stage, and all plasmacytomas. Abbreviations: ESS, effective sample size.
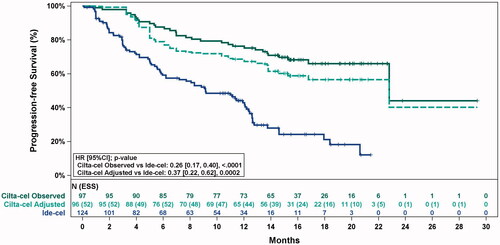
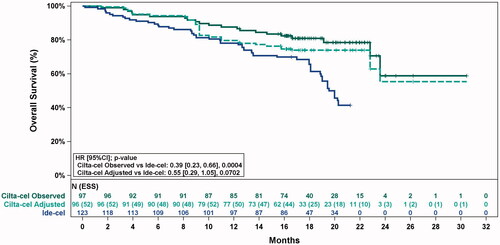
Figure 3. Observed (unadjusted) and adjusted (base case) Kaplan–Meier plots of overall survival. Note. Base case results adjusted for refractory status, cytogenetic profile, revised International Staging System stage, and all plasmacytomas. Abbreviations: ESS, effective sample size.
Supplemental Material: Appendix
Download ()Data availability statement
Requests for access to the CARTITUDE-1 trial study data may be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu. The data sharing policy of Janssen Pharmaceutical Companies is available at https://www.janssen.com/clinical-trials/transparency. For KarMMa study, a data sharing statement is available with the full text of the primary publication at NEJM.orgCitation10.
