Figures & data
Table 1. Design of trials included in this study.
Table 2. Comparison of patient eligibility criteria for included trials.
Figure 1. Distribution of baseline patient characteristics across trials in different MS phenotypes: EXPAND (SPMS), FREEDOMS (RRMS), and INFORMS (PPMS). Note: The INFORMS trial was not included in the number of relapses plots because the PPMS patients did not have relapses. For the time since first MS symptom and time since MS diagnosis, comparisons with INFORMS (PPMS) may be biased by the systematic underestimation of MS duration in PPMS patients (who typically have accumulated lesions but not acute neurological events). Additional comparisons between trials included in this study can be found in Supplemental Appendix B. Abbreviations. EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; PPMS: primary progressive multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis.
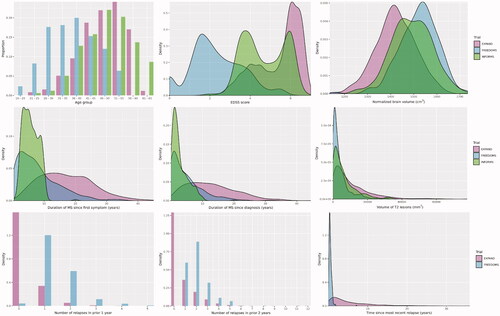
Table 3. Published patient baseline characteristics for the siponimod, fingolimod (0.5 mg), or ofatumumab treatment arm of included trials.
Table 4. Summary of trial-specific outcome definitions.
Figure 2. Time to confirmed disability progression in placebo arms of the EXPAND, FREEDOMS, FREEDOMS II, and INFORMS trials. Note: The shaded areas of the plot that surround the curves represent the 95% confidence intervals around the point estimates at each time point. Curves were derived from IPD for the placebo arms of the indicated trials: EXPAND (SPMS), FREEDOMS (RRMS), FREEDOMS II (RRMS), and INFORMS (PPMS). Curves were generated before excluding relapsing patients from EXPAND and before conducting PSM on treatment arms (EXPAND and INFORMS). The TRANSFORMS (in RRMS) and ASCLEPIOS I/II (in RMS) trials were not included because they were not placebo-controlled. Abbreviations. 3mCDP: 3-month confirmed disability progression; 6mCDP: 6-month confirmed disability progression; IPD: individual patient data; PPMS: primary progressive multiple sclerosis; PSM: propensity score matching; RMS: relapsing multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis.
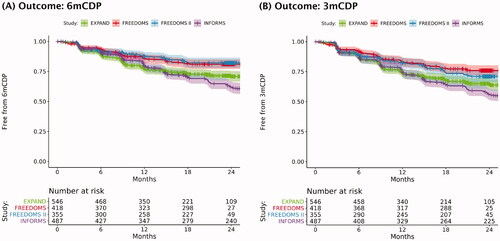
Table 5. Propensity score matching analysis results.
