Figures & data
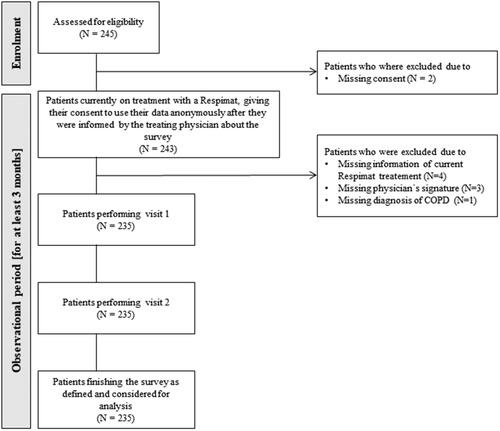
Figure 1. Training material used for patients´ education. Overview about the training material provided by Boehringer for the launch of the reusable Respimat which was used in the current survey for patients´ education. (A) Placebo inhaler. (B) Patient brochure (partly shown). (C) Video cards/demo films (partly shown). (D) SMS reminder service (PATSU). Abbreviation. PATSU, PATient SUpport service.
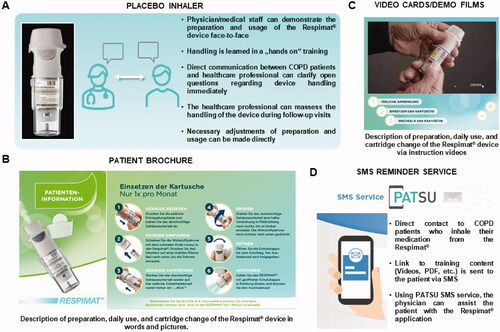
Table 1. Patient demographics, COPD characteristics and current inhaled therapy for COPD treatment (all patients, N = 235).
Table 2. Concomitant diseases with effect on handling and ability to inhale with the Respimat inhaler and further comorbidities (all patients, N = 235).
Figure 3. Patients´ evaluation of the new reusable Respimat inhaler (all patients, N = 234*). Patients were asked to evaluate the new reusable Respimat inhaler with focus on dose counter (A), monthly preparation (B), handling during daily use (C) after changing at least once the cartridges at home, and overall satisfaction (D). Further they were asked how important environmental friendliness is to them (E). Shown is the number of patients who chose the respective answer. Percentages are given in brackets. *1 Patient was excluded due to missing signature of the respective physician at visit 2.
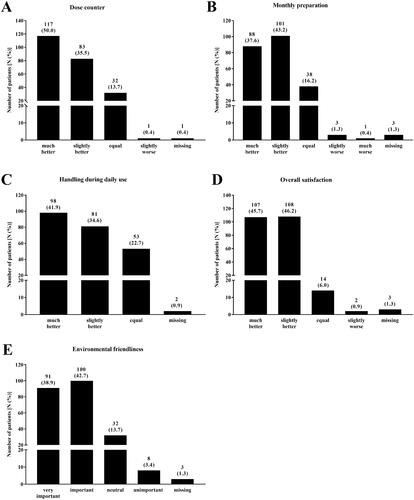
Figure 4. Medical evaluation of the new reusable Respimat compared to the previous version (all patients, N = 235). Physicians were asked to report the ability of the patient to use the new reusable Respimat inhaler (A) and whether the patients need further training (B) after changing the cartridges at least once at home. (A) Given is the number of patients (percentages are given in brackets). (B) Given is the number of patients in %. Abbreviation. N, number of patients.
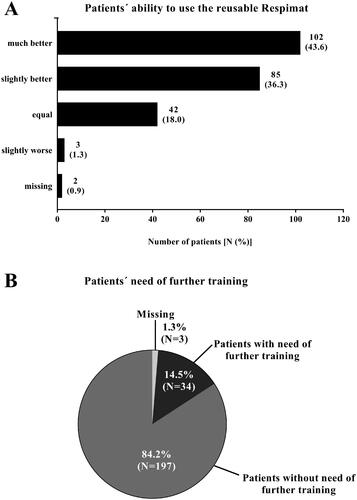
Figure 5. Physicians´ and patients´ preferred training material (all patients, N = 235). Physicians and patients were asked for their preferred training material to demonstrate the correct usage of the Respimat inhaler and to learn how to use it (A). Given is the number of physicians as well as the number of patients who preferred the respective training material. (B) Physicians´ report which training material was used for patient´s re-training. Percentages are given in brackets. Abbreviation. N, number of patients.
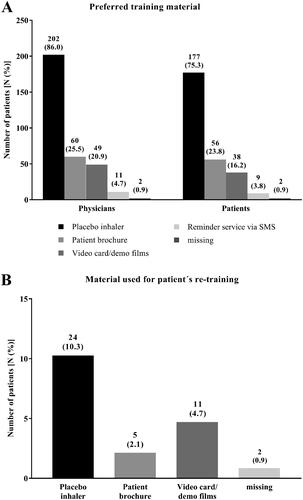
Table 3. Subgroup analysis: patients with concomitant diseases affecting the handling of the Respimat inhaler and the ability to inhale (grouped, N = 86).
Figure 6. Subgroup analysis – patients with concomitant diseases effecting the handling of the Respimat inhaler and the ability to inhale: Patients´ evaluation of the new reusable Respimat inhaler (N = 85*). Graphs show the patients´ evaluation of dose counter (A), handling during daily use (B), monthly preparation (C), and overall satisfaction (D) with the Respimat inhaler after they had changed 1–3 cartridges at home using a scale with pre-defined answers (much better, slightly better, equal, slightly worse, and much worse). Given is the percentage of patients who chose the respective answer. *1 Patient in Groupmob was excluded due to missing signature of the respective physician at visit 2. Abbreviations. N, number of patients; mob, Group mobility; cog, group cognitive disorders; both, group mobility and cognitive disorders.
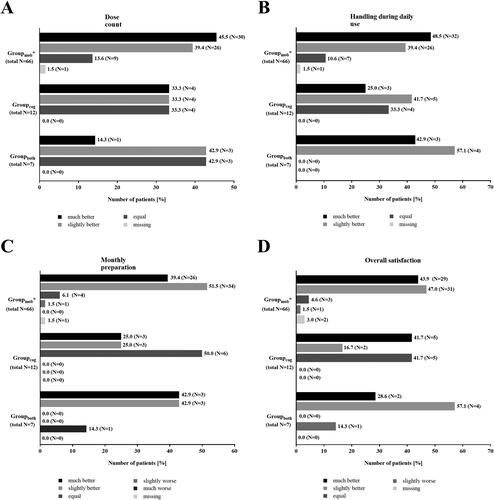
Figure 7. Subgroup analysis – Patients with concomitant diseases effecting the handling of the Respimat inhaler and the ability to inhale (N = 86): Preferred training material – physicians vs. patients. Physicians and patients were asked for their preferred training material to demonstrate the correct usage of the Respimat inhaler and to learn how to use it. Given is the percentage of patients for whom the physician has favored the respective training option (A) as well as the number of patients who preferred the respective training material (B) in regard with their concomitant disease. Abbreviations. N, number of patients; mob, group mobility; cog, group cognitive disorders; both, group mobility and cognitive disorders.
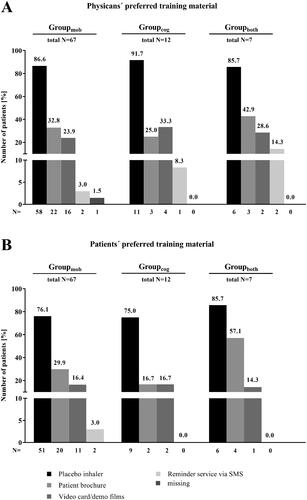
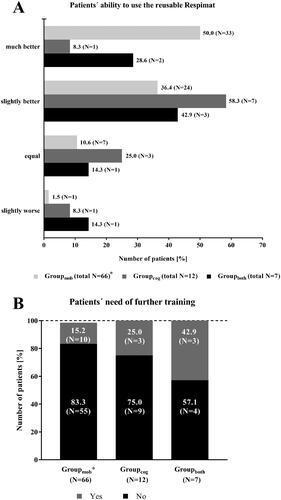
Figure 8. Subgroup analysis – Patients with concomitant diseases effecting the handling of the Respimat inhaler and the ability to inhale: Medical evaluation of the new reusable Respimat compared to the previous version (N = 85). Physicians were asked to report the ability of the patient to use the new reusable Respimat inhaler (A) and whether the patients need further training (B) after changing the cartridges at least once and maximum 3 times at home. *1 Patient in Groupmob was excluded due to missing signature of the respective physician at visit 2. Abbreviations. N, number of patients; mob, group mobility; cog, group cognitive disorders; both, group mobility and cognitive disorders.
Supplementary_Material_1_NTA_Switch.pdf
Download PDF (381.5 KB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.