Figures & data
Table 1. Comparison of endpoint definitions.
Table 2. Overview of study baseline characteristics before and after matching (clinical and hematological endpoints).
Table 3. Overview of study baseline characteristics before and after matching (fatigue and quality of life endpoints).
Figure 1. Anchored comparisons of clinical and hematological endpoints through Week 16 (PEGASUS study) and Week 26 (ALXN1210-PNH-302 study) after matchinga. Abbreviations. CI: confidence interval; LDH, lactate dehydrogenase; SD, standard deviation. Notes. aThe following baseline characteristics were matched on: age at first infusion of study drug, female, White, Asian, history of aplastic anemia, and LDH. bChange from baseline in LDH level was examined for Week 16 (Day 113) for the ALXN1210-PNH-302 study. Baseline mean and SD for LDH level were reported in of the Kulasekararaj et al.Citation10 publication. Week 16 (Day 113) mean and 95% CI for LDH level were extracted from Supplemental Figure S3 of the Kulasekararaj et al.Citation10 publication. The SD for LDH level at Week 16 (Day 113) was calculated using the following equation: √(N)*(upper limit of CI – lower limit of CI)/3.92. During follow-up, LDH level was available at Week 16 (Day 113) for 94 patients who received ravulizumab and 96 patients who received eculizumab in the ALXN1210-PNH-302 study. cIn the PEGASUS study, LDH level normalization is defined as the proportion of patients who achieved LDH level ≤1× ULN (226 U/L) in the absence of transfusions from baseline through the end of follow-up. In the ALXN1210-PNH-302 study, LDH level normalization is defined as the proportion of patients who achieved LDH level ≤1× ULN (246 U/L), with or without transfusions. Week 16 (Day 113) mean and 95% CI for the proportion of patients with LDH level normalization in the ALXN1210-PNH-302 study were extracted from of the Kulasekararaj et al. Citation10 publication.
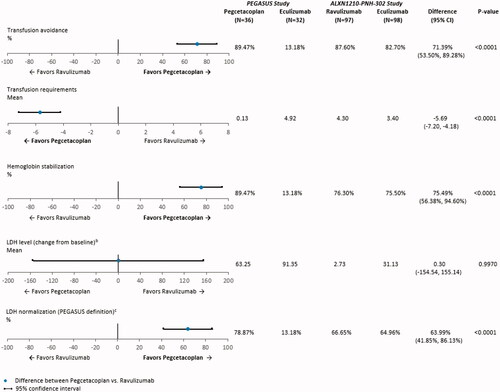
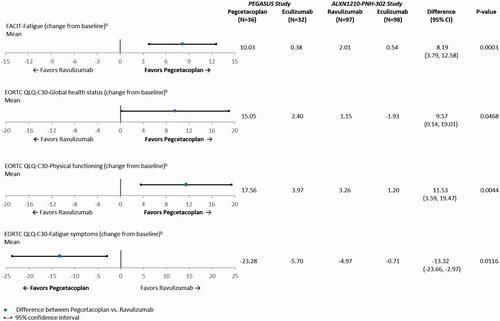
Figure 2. Anchored comparisons of fatigue and quality of life endpoints through Week 16 (PEGASUS study) and Week 26 (ALXN1210-PNH-302 study) after matchinga. Abbreviations. CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; FACIT, Functional Assessment of Chronic Illness Therapy; LDH, lactate dehydrogenase. Notes. aThe following baseline characteristics were matched on: age at first infusion of study drug, weight, history of aplastic anemia, and LDH. bData were available for 67 of 68 patients in the PEGASUS study.
REVISED_SUPP_MAT__clean_-Bhak-Manuscript-CMRO-MAIC_of_pegcetacoplan_in_second-line_PNH-07.19.2021.docx
Download MS Word (57.4 KB)Data availability statement
For data requests, please contact Rachel H. Bhak ([email protected]).
