Figures & data
Table 1. Study characteristics.
Figure 1. Flowchart of included citations. Abbreviations. ASA, Acetylsalicylic acid; CAD, Coronary artery disease; PAD, Peripheral artery disease; RCT, Randomized controlled trial.
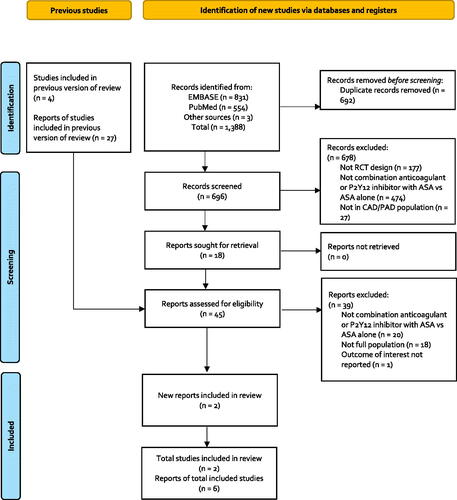
Figure 2. Diagram of the indirect treatment comparison. Abbreviations. CHARISMA, Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; ITC, Indirect treatment comparison.
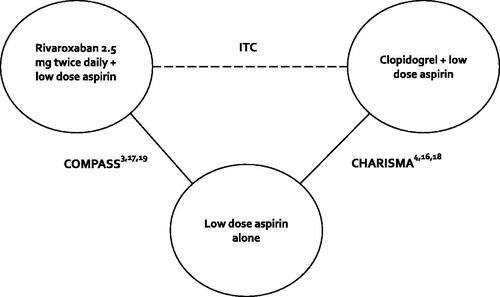
Figure 3. RoB assessment. Abbreviations. R, Risk arising from the randomization process; D, Bias due to deviations from intended interventions; Mi, Bias due to missing outcome data; Me, Bias in measurement of the outcome; S, Bias in selection of the reported result; O, Overall risk of bias; CHARISMA, Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; RoB, Risk of bias.
Figure 4. Results of efficacy outcomes for the indirect treatment comparison. Abbreviations. CI, Confidence interval; CV, Cardiovascular; HR, Hazard ratio; ICH, Intracranial hemorrhage; MACE, Major adverse cardiovascular event; MI, Myocardial infarction; RR, Risk ratio.
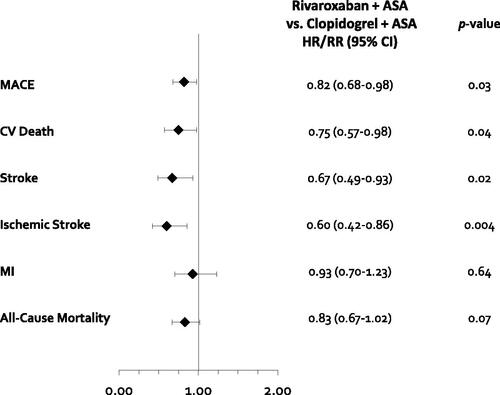
Table 2. Results of efficacy outcomes for the indirect treatment comparison.
Figure 5. Results of safety outcomes for the indirect treatment comparison. Abbreviations. CI, Confidence interval; CV, Cardiovascular; HR, Hazard ratio; ICH, Intracranial hemorrhage; MACE, Major adverse cardiovascular event; MI, Myocardial infarction; RR, Risk ratio. *Calculated using moderate/severe bleeding per clinician evaluation collected from Eikelboom et al., Citation17.
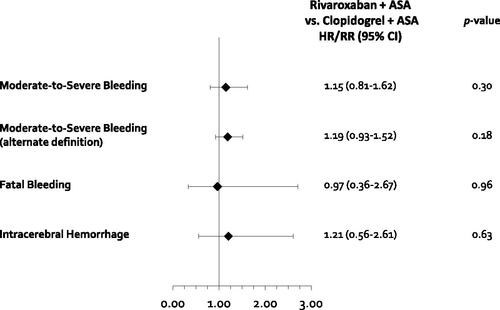
Table 3. Results of safety outcomes for the indirect treatment comparison.
