Figures & data
Figure 1. Cohort Selection. 1CRC screening tests were mt-sDNA, colonoscopy, fecal immunochemical testing (FIT), fecal occult blood testing (FOBT), sigmoidoscopy, CT colonography and double-contrast barium enema (DCBE). 2FIT or FOBT in the year prior to the index date, no evidence of mt-sDNA test in 3 years prior to the index date, no evidence of other screening (sigmoidoscopy, CT colonography or DCBE) in 5 years prior to the index date, and no evidence of colonoscopy in 10 years prior to the index date. 3Codes and conditions for identifying above average-risk adults can be found in Supplementary Table 1.
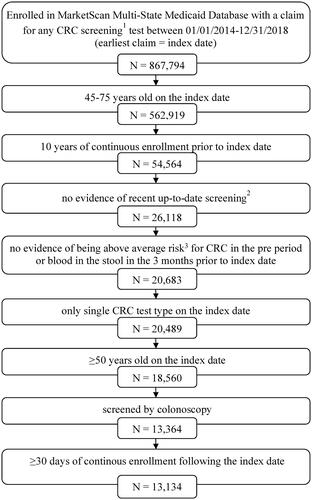
Table 1. Patient characteristics of average-risk adults with screening colonoscopy covered by Medicaid insurance.
Table 2. Utilization and costs associated with screening colonoscopy in the 30 days prior to and following colonoscopy among average-risk adults covered by Medicaid insurance.
Table 3. Gastrointestinal (GI) and cardiovascular/cerebrovascular (CV) events and costs associated with eventsa in the 30 days following colonoscopy among average-risk adults covered by Medicaid insurance.
Supplemental Material
Download MS Word (58 KB)Data availability statement
The data that support the findings of this study are available from IBM Watson Health. Restrictions apply to the availability of these data, which were used under license for this study.
