Figures & data
Table 1. Objectives and cohort level outcomes in CLARION.
Table 2. Planned data sources, population coverage, and data elements to be included in CLARION.
Table 3. Additional exposure definitions.
Figure 1. Overview of data flow from collection to the consolidated database in the CLARION study. CRO, clinical research organization; MSDS 3D, Multiple Sclerosis management system 3 Dimension; MSBase, Multiple Sclerosis database: NTD, NeuroTransData database; OFSEP, Observatoire Français de la Sclérose en Plaques; SMSC, Swish MS Cohort.
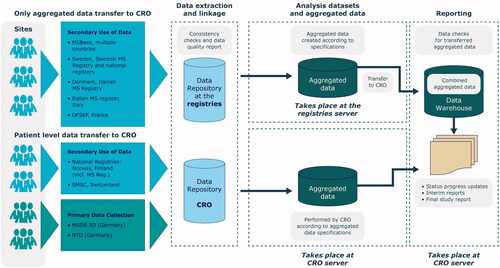
Table 4. Overview of planned analyses.
Figure 2. Inclusion status in the CLARION study over time. The figure shows the number of patients included in the study at different data cut-offs, and this is regardless of the initial treatment time (some patients added in 2020 were already treated in 2019).
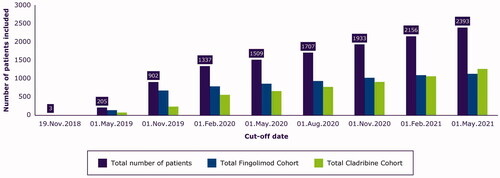
Table 5. Registry participation in the CLARION study, over time.
Table 6. Demographics and disease characteristics of patients in CLARION (cut-off date: 1 May 2021).
