Figures & data
Figure 1. Overall study period. Abbreviations. CDK, cyclin-dependent kinase; HER2, human epidermal growth factor receptor; HR, hormone receptor. *Index date defined as the date of earliest CDK4 and 6 inhibitor-containing regimen during this period.
Figure 2. Overall study (A) and chart review (B) attrition. Abbreviations. CDK4 and 6, cyclin-dependent kinase 4 and 6 inhibitor; HER2−, human epidermal growth factor receptor 2-negative; HR+, hormone receptor positive; N, population size; n, sample size. aIncluded receipt of treatment indicated for another primary cancer prior to or during the study observation period. bTargeted sample selection described in the Methods section. cInitial inclusion/exclusion criteria examined during structured data review were re-examined in detailed chart review to ensure accuracy. dCategories not mutually exclusive. eA targeted sample selection was implemented to identify all available patients receiving abemaciclib and ribociclib, and a randomly selected sample of patients receiving palbociclib; this sampling strategy was defined a priori with the goal of achieving approximately equal sample sizes across treatment groups and was selected due to variations in post-approval periods leading to differences in utilization.
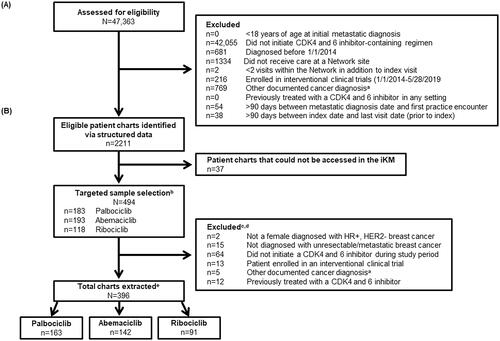
Table 1. Baseline demographic and clinical characteristics of female patients with HR+, HER2− MBC receiving a CDK4 and 6 inhibitor in a US community patient population.
Table 2. Dosing schedule of CDK4 and 6 inhibitor-containing regimens.
Figure 3. Median (interquartile range) time-to-onset of the most frequently occurring select adverse events* in the overall cohort and by CDK4 and 6 inhibitor. Abbreviations. CDK, cyclin-dependent kinase 4 and 6 inhibitor; HER−, human epidermal growth factor receptor 2-negative; HR+, hormone receptor positive; MBC, metastatic breast cancer; n, sample size.
*Adverse events that occurred in <5 patients within any cohort are not presented in this figure. Vertical lines drawn at 50 and 100 days for ease of interpretation.
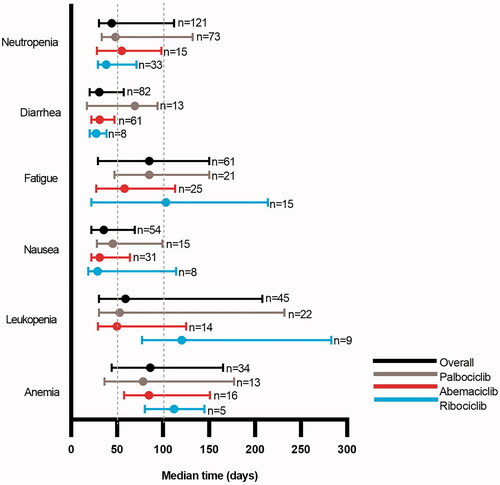
Table 3. Adverse events of interest (all-grade) for those AEs occurring in ≥5% of the overall cohort, and associated outcomes of female patients with HR+, HER2− MBC receiving a CDK4 and 6 inhibitor.
Table 4. Healthcare resource utilization due to AEs of interest during index CDK4 and 6 inhibitor treatment.
Price_et_al_Supplementary_Materials.docx
Download MS Word (15.1 KB)Data availability statement
The health data used to support the findings of this study are restricted by the US Oncology Institutional Review Board to protect patient privacy. For this reason, data used to support the findings of this study have not been made available.
