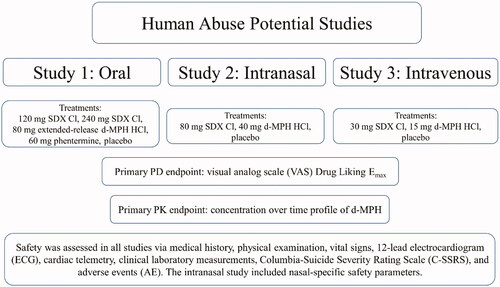
Figures & data
Figure 1. Serdexmethylphenidate chemical structure. SDX consists of a single d-MPH molecule covalently attached via a carbamate bond to a methylene oxide linker, which in turn is connected to a nicotinoyl-serine moiety. Molecular components: red = d-methylphenidate, black = carboxymethylene linker, blue = niacin, green = l-serine.
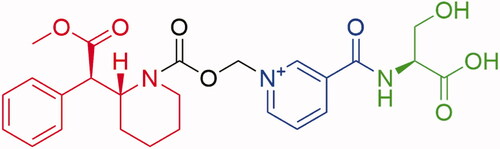
Table 1. Study participant demographics (Completers population).
Figure 3. Mean Drug Liking VAS over time (top left) and Emax values for primary endpoint (Drug Liking, top right) and secondary endpoints (Take Drug Again, Overall Drug Liking, bottom) following oral (po) administration of SDX Cl (120 and 240 mg), extended-release d-MPH (80 mg), phentermine (60 mg), and placebo. Raw means are shown for Emax scores. Treatment comparisons were performed with a mixed-effect ANCOVA model as described in the “Statistical analyses” section.
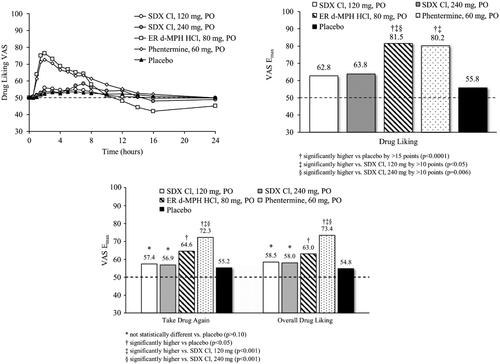
Table 2. Summary results for mean Emax of secondary VAS endpoints for study 1 (oral administration), study 2 (IN administration), and study 3 (IV administration).
Figure 4. Plasma d-MPH concentrations after oral (PO) administration of SDX Cl and ER d-MPH HCl. Bars are standard deviations.
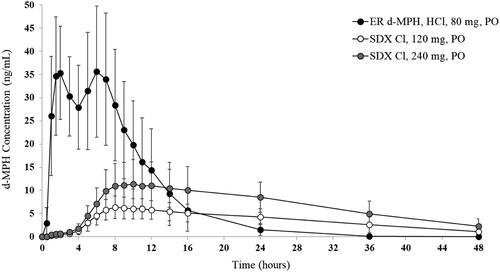
Figure 5. Mean Drug Liking VAS over time (top left) and Emax values for primary endpoint (Drug Liking, top right) and secondary endpoints (Take Drug Again, Overall Drug Liking, bottom) following intranasal (IN) administration of SDX Cl (80 mg), d-MPH HCl (40 mg), and placebo. Raw means are shown for Emax scores. Pairwise comparison of d-MPH HCl and placebo was performed using the Sign test. The remaining treatment comparisons were conducted with a matched-pairs t-test. The detailed test methodologies are described in the “Statistical Analyses” section.
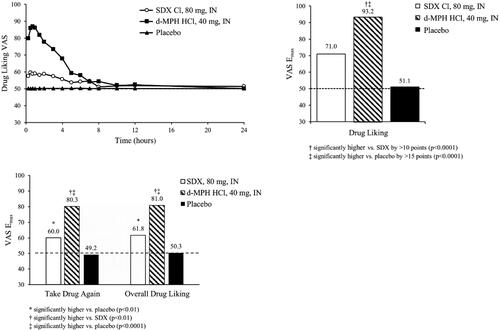
Figure 6. Plasma d-MPH concentrations after intranasal (IN) administration of SDX Cl and d-MPH HCl. Bars are standard deviations.
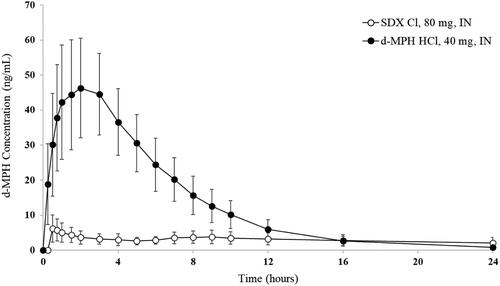
Figure 7. Mean Drug Liking VAS over time (top left) and Emax values for primary endpoint (Drug Liking, top right) and secondary endpoints (Take Drug Again, Overall Drug Liking, bottom) following intravenous (IV) administration of SDX Cl (30 mg), d-MPH HCl (15 mg), and placebo. Raw means are shown for Emax scores. Matched-pairs t-tests were performed for the comparisons of Drug Liking Emax and Overall Drug Liking Emax between d-MPH HCl and placebo, and Overall Drug Liking Emax between SDX Cl and placebo. Pairwise comparisons of Drug Liking Emax and Overall Drug Liking Emax between d-MPH HCl and SDX Cl, and Drug Liking Emax between SDX Cl and placebo were conducted using the Sign test. All treatment comparisons of Take Drug Again Emax were performed with a mixed-effect ANCOVA model. The detailed test methodologies are described in the “Statistical Analyses” section. Note that Take Drug Again was assessed on a unipolar scale.
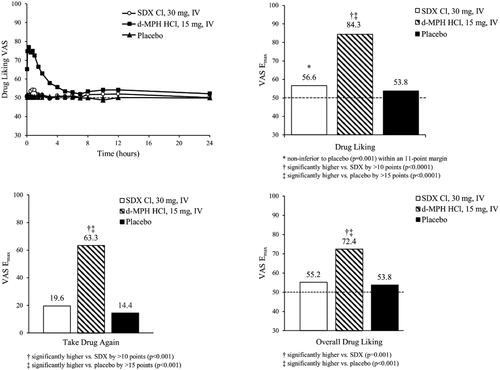
Figure 8. Plasma d-MPH concentrations after intravenous (IV) administration of SDX Cl and d-MPH HCl. Bars are standard deviations.
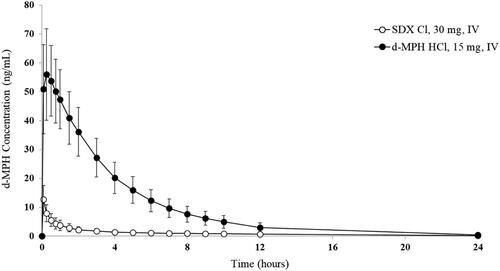
Table 3. Treatment-emergent adverse events reported by >20% of subjects (for any treatment).