Figures & data
Table 1. Acute migraine: selected guidelines.
Table 2. Knee OA: selected guidelines.
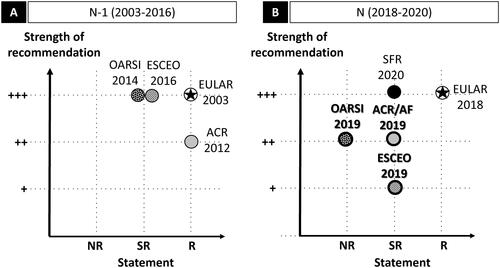
Figure 1. Acute migraine: graphic representation of paracetamol recommendations according to the statement and the strength of the recommendation. Abbreviations. AAN, American Academy of Neurology; AHS, American Headache Society; CHS, Canadian Headache Society; EFNS, European Federation of Neurological Societies; EHF, European Headache Federation; LTB, Lifting The Burden; N, current recommendation; N − 1, previous recommendation; NR, non-recommended; R, recommended; SFEMC, Société Française d’Etudes des Migraines et Céphalées; SR, specific recommendation. Panel A and panel B graphically present the position of the N − 1 and N recommendations respectively, with statement in the x-axis, and the strength of the recommendations in the y-axis. For the x-axis, recommendations are classified in three levels: NR, SR, and R. SR means that the paracetamol may be used under certain specific conditions. The specific conditions are as follows: for the EHF/LTB (both 2007 and 2009), paracetamol is recommended if NSAIDs are contraindicated. For the y-axis, the three levels range from weak (+) to strong (+++). The EHF/LTB did not mention a strength for their recommendation (and level of evidence), in this case the recommendation is considered +++ by default. The AHS 2015 did not mention a strength for their recommendation but had a level of evidence (level A for “non-incapacitating attacks: paracetamol 1000 mg”), in this case the recommendation was considered +++ by default. For more details, see Supplementary Table 1d.
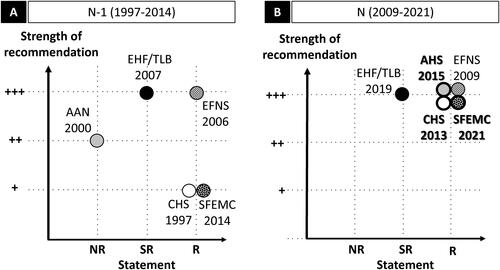
Figure 2. Knee OA: graphic representation of paracetamol recommendations according to the statement and the strength of the recommendation. Abbreviations. ACR/AF, American College of Rheumatology/Arthritis Foundation; ESCEO, European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases; EULAR, European League Against Rheumatism; N, current recommendation; N − 1, previous recommendation; NR, non-recommended; OARSI, Osteoarthritis Research Society International; R, recommended; SFR, Société Française de Rhumatologie; SR, specific recommendation. Panel A and panel B graphically present the position of the N − 1 and N recommendations, respectively, with the statement in the x-axis, and the strength of the recommendation in the y-axis. For the x-axis, recommendations are classified in three levels: NR, SR, and R. SR means that the paracetamol may be used under certain specific conditions. The specific conditions are as follows: for the OARSI 2014, paracetamol is appropriate for OA patients without comorbidity, at conservative dose and treatment duration consistent with approved prescribing limits; for the ESCEO (both 2016 and 2019), paracetamol is recommended as short-term rescue analgesia in step 1; for the ACR/AF 2019, paracetamol is recommended for short-term and episodic use for intolerance or contraindication to NSAIDs; for the SFR 2020, paracetamol is not recommended for systematic and/or continuous prescription. Star (★) indicates that paracetamol is prescribed as first-line treatment. For the y-axis, the three levels range from weak (+) to strong (+++). For the ESCEO 2016 (N − 1) which did not mention a strength (and level of evidence), the recommendation is considered +++ by default. No previous guideline (N − 1) for knee OA exists for the SFR; for the EULAR, no re-evaluation has been performed since 2003 (N − 1). The ESCEO developed a stepwise algorithm and stratified knee OA according to pharmacological treatment and severity (Step 1: background treatment; Step 2: advanced pharmacological treatment, Step 3: last pharmacological attempts, Step 4: end-stage disease management and surgery). The OARSI defined patient subgroups (“no comorbidities”, “gastrointestinal comorbidities”, “cardiovascular comorbidities”, “frailty”, and “widespread and pain/or depression”). For more details, see Supplementary Table 2d.