Figures & data
Figure 1. Patient attrition chart for the selection of patients with advanced/recurrent endometrial cancer who were treated with a first-line platinum-based chemotherapy and initiated second-line anti-neoplastic therapy. aPatients must not have any record of treatment with an anti-neoplastic agent (excepting hormonal agent) in the 12 months before 1L treatment initiation. bPlatinum-based regimens included carboplatin, cisplatin, and oxaliplatin. cInitiation of 2L treatment with a chemotherapy, immunotherapy, or targeted therapy agent. dEndometrial cancer-related surgery included hysterectomy (subtotal, total, radical, or unspecified), salpingo-oophorectomy, or total hysterectomy + bilateral salpingo-oophorectomy + omentectomy with or without lymphadenectomy including sentinel lymph node dissection. Abbreviations. 1L, first-line; 2L, second-line; ICD-9-CM/ICD-10-CM: International Classification of Disease, Ninth/Tenth Revision, Clinical Modification.
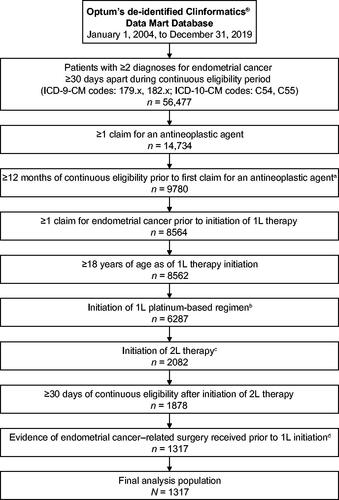
Table 1. Demographic and clinical characteristics prior to the initiation of each line of therapy.
Figure 2. Treatment sequence for recurrent, metastatic, or high-risk endometrial cancer in (A) the overall population (N = 1317) and (B) patients who were indexed in 2018 or 2019 (N = 163). Each ring represents one line (L) of therapy, starting with first-line treatment in the innermost ring. Abbreviations. 1L, first-line; 2L, second-line; 3L, third-line; 4L, fourth-line; PD-1, programmed death 1; PD-L1, programmed death ligand 1.
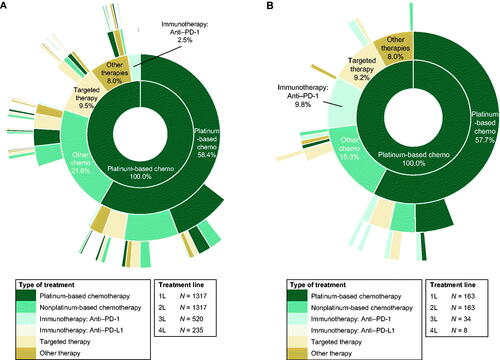
Table 2. Duration and type of treatment for advanced or recurrent endometrial cancer, overall population.
Table 3. Duration and type of treatment for advanced or recurrent endometrial cancer, patients with index date in 2018 or 2019.
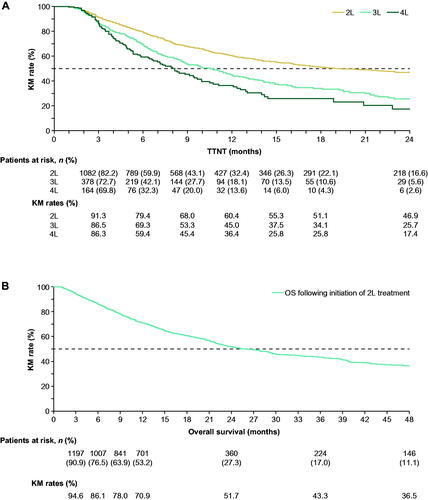
Figure 3. (A) TTNT and (B) overall survival in the overall population. TTNT was measured as the time from the initiation of a line of therapy to the date of initiation of the next line of therapy. Patients who did not initiate a next line of therapy were censored at the end of continuous eligibility, death, loss to follow-up, or end of data availability, whichever occurred first. Overall survival was defined as the time between the initiation of second-line treatment and the date of death. Patients alive at the end of their eligibility period were censored. Abbreviations. 2L, second-line; 3L, third-line; 4L, fourth-line; KM, Kaplan–Meier; TTNT, time to next treatment.
TxPatternsECOptMs_CMRO_19Jan2022_Suppl_Materials.docx
Download MS Word (52.7 KB)Data availability statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
