Figures & data
Thoracic endovascular aortic repair of a large, multilobulated, calcified thoracic aneurysm. Read the transcript
Figure 1. Proposed systematic approach for diagnosing and managing patients with osteoporosis to prevent osteoporotic fractures.
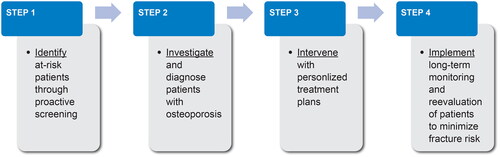
Figure 2. Questions to ask during routine clinic visits to identify patients with or at risk for osteoporosis and osteoporotic fractures.
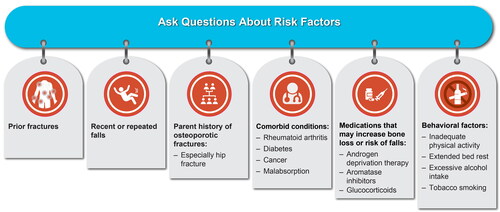
Figure 3. Patients who warrant BMD testing. aA BMD scan is performed by DXA and provides a T-score (derived by comparing a patient’s BMD values [in g/cm2] with those from a uniform Caucasian female normative database; the International Society of Clinical Densitometry recommends the normative database be used for women of all ethnic groups and a Caucasian female reference group be used for men of all ethnic groups). bLoss of height should ideally be measured with a stadiometer. cDefined as the inability to touch the back of the head to the wall when standing with back and heels against the wall. dTimed up-and-go (TUG) test assesses mobility, balance, walking ability, and fall risk in older adults https://www.cdc.gov/steadi/pdf/TUG_Test-print.pdf [cited 2022 Feb 19]. Briefly, to perform the test, (1) Begin by having the patient sit back in a standard armchair and identify a line 3 m (10 ft) away on the floor and (2) On the word “Go,” record the time it takes for the patient to rise from the chair, walk to the line, and return to the chair, and sit down. An older adult who takes ≥ 12 s to complete the TUG is at risk for falling. BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry.
![Figure 3. Patients who warrant BMD testing. aA BMD scan is performed by DXA and provides a T-score (derived by comparing a patient’s BMD values [in g/cm2] with those from a uniform Caucasian female normative database; the International Society of Clinical Densitometry recommends the normative database be used for women of all ethnic groups and a Caucasian female reference group be used for men of all ethnic groups). bLoss of height should ideally be measured with a stadiometer. cDefined as the inability to touch the back of the head to the wall when standing with back and heels against the wall. dTimed up-and-go (TUG) test assesses mobility, balance, walking ability, and fall risk in older adults https://www.cdc.gov/steadi/pdf/TUG_Test-print.pdf [cited 2022 Feb 19]. Briefly, to perform the test, (1) Begin by having the patient sit back in a standard armchair and identify a line 3 m (10 ft) away on the floor and (2) On the word “Go,” record the time it takes for the patient to rise from the chair, walk to the line, and return to the chair, and sit down. An older adult who takes ≥ 12 s to complete the TUG is at risk for falling. BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry.](/cms/asset/580d690c-78c9-4dfe-87c0-51085ce23082/icmo_a_2141483_f0003_c.jpg)
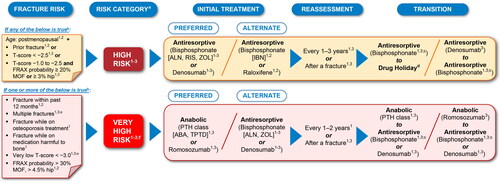
Figure 4. Common elements in fracture risk guidelines and treatment strategies for management of postmenopausal osteoporosis by risk categorization. The recommendations summarized here are a consolidation of recommendations across current clinical guidelines presented in a simplified manner and streamlined for a global audience. Sources: (1) Camacho et al.Citation4; (2) Kanis et al.Citation6 and Kanis et al.Citation43; and (3) Shoback D et al.Citation7 and Eastell et al.Citation68 Routes of administration by drug class: the bisphosphonates ALN, IBN, and RIS are administered as oral tablets; IBN is also available as intravenous injection in several countries; the bisphosphonate ZOL is administered by infusion; denosumab is administered by subcutaneous injection; PTH class of drugs ABA and TPTD are administered by subcutaneous injection; raloxifene is administered as an oral tablet, and romosozumab is administered by subcutaneous injection. Of note, bisphosphonate antiresportive agents (ALN, IBN, RIS, and ZOL) have a different mechanism of action from the other antiresorptive agent denosumab, and it is important that clinicians understand the differential bone effects between these two types of antiresorptive agents. aRegional and local guidelines may override some of these criteria based on differences in FRAX data and cost-effectiveness thresholds. bIf FRAX is not available, major determinants of risk should include age, BMD, fractures, and medication harmful to bone. cOff-treatment period for consideration after treatment with bisphosphonates for 3–5 years due to BMD gains plateauing at ∼3 years. dApplicable if decision is made to discontinue denosumab. eENDO requires both risk factors of multiple fractures and very low T-score < –3.0 to be met for very high risk categorization. fVery high risk category includes the imminent risk subcategory that refers to the relative risk of recurrent fracture that is highest in the first years following an index fracture in patients. ABA, abaloparatide; ALN, alendronate; BMD, bone mineral density; ENDO, Endocrine Society; FRAX, Fracture Risk Assessment Tool; IBN, ibandronate; MOF, major osteoporotic fracture; PTH, parathyroid hormone; RIS, risedronate; TPTD, teriparatide; ZOL, zoledronic acid.
Supplemental Material
Download MS Word (5.3 MB)Data availability statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request/.
