Figures & data
Table 1. Baseline demographic and lifestyle characteristics of the Latin American study population (N = 461).
Table 2. Disease characteristics of the Latin American study population (N = 461).
Figure 1. Differences in the number of severe exacerbations evaluated using the ANOVA test across the Latin American study population with atopic phenotype (total serum IgE >100 IU/mL and BEC <300 cells/mm3) and with eosinophilic phenotype (total serum IgE ≤100 IU/mL and BEC ≥300 cells/mm3). Abbreviations. ANOVA, analysis of variance; IgE, immunoglobulin E.
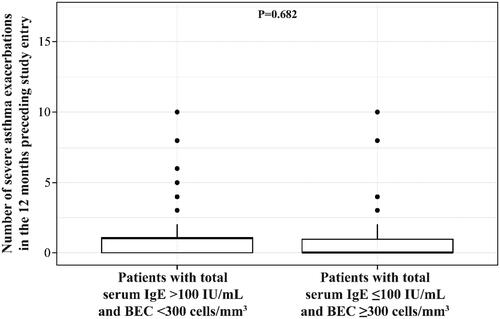
Table 3. Hematological assessments in the Latin American study population (N = 461).
Table 4. Baseline demographic and lifestyle characteristics of the Latin American study population (N = 461) by BECs and serum IgE concentrations levels.
Table 5. Disease characteristics of the Latin American study population (N = 461) by BECs and serum IgE concentrations levels.
Table 6. Pattern of OCS use by BECs and serum IgE concentrations in the Latin American study population (N = 461).
Table 7. Asthma treatments prescribed in the previous 12 months to the Latin American study population (N = 461).
Table 8. Asthma symptom control in the Latin American study population (N = 461).
PREPARE_manuscript_supplemental_file.docx
Download MS Word (26.5 KB)Data availability statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
