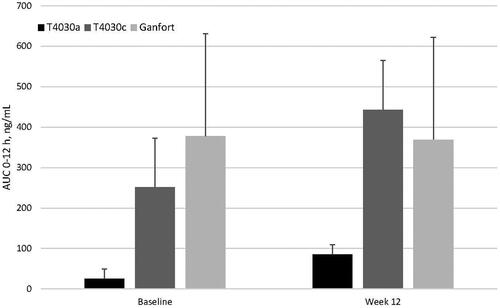
Figures & data
Figure 1. Flow chart of patient disposition. Abbreviations. ITT, intent-to-treat; mITT, modified intent-to-treat; PP, perprotocol; PK, pharmacokinetics. A one patient performed screening visit but withdrew from the study before the randomisation visit due to abnormal ECG. The patient was considered a screen failure but was randomised incorrectly in the Ganfort group. As only the screening visit was performed, the patient was excluded from the study. Consequently, the total number of patients considered in the study was 86.
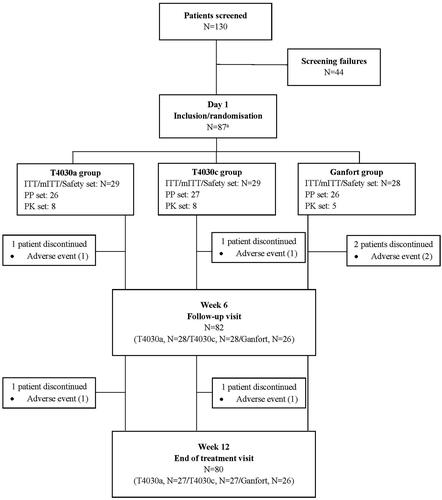
Table 1. Demographic and other characteristics at Screening (mITT set).
Table 2. IOP (mmHg) in the worse eye at baseline (day 1; 08:00), week 6 and week 12 (primary efficacy analysis) and change from baseline (mITT Set).
Table 3. Summary of AEs related to treatment (Safety set).