Figures & data
Figure 1. Patient disposition. aIncludes patients with schizophrenia or BP-I. Data for the subpopulation of patients with schizophrenia will be reported elsewhereCitation18. bCompleted visit on Day 225. Abbreviations. AE, adverse event; AOM 400, aripiprazole once-monthly 400 mg; Ari 2MRTU 960, aripiprazole 2-month ready-to-use 960 mg; BP-I, bipolar I disorder.
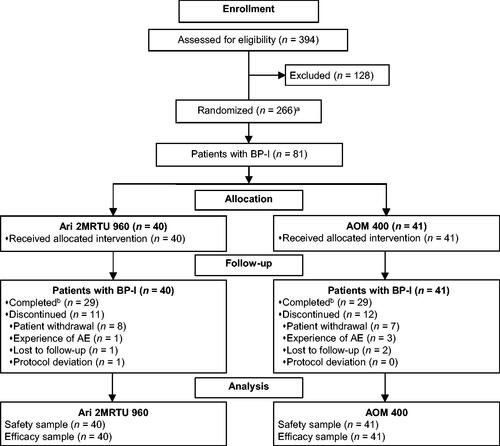
Table 1. Demographics and baseline disease characteristics in patients with BP-I.
Table 2. Summary of TEAEs in patients with BP-I.
Figure 2. Efficacy outcomes at Week 32. Data shown are from the efficacy analysis sample (MMRM analysis). Mean (SD) YMRS Total score at baseline was 6.6 (7.4) in the Ari 2MRTU 960 group and 9.4 (8.3) in the AOM 400 group. Mean (SD) MADRS Total score at baseline was 11.0 (9.5) in the Ari 2MRTU 960 group and 13.0 (9.3) in the AOM 400 group. Mean (SD) CGI-BP score at baseline was 2.3 (1.2) in the Ari 2MRTU 960 group and 2.8 (1.2) in the AOM 400 group. Mean (SD) SWN-S score at baseline was 91.9 (17.3) in the Ari 2MRTU 960 group and 89.2 (18.5) in the AOM 400 group. ap-values for between-group comparison were derived from an MMRM analysis with fixed effects of treatment, pharmacokinetic sampling schedule for determining the concentration of aripiprazole in patients’ plasma, week, treatment-by-week interaction, and baseline-by-week interaction as covariate. Abbreviations. AOM 400, aripiprazole once-monthly 400 mg; Ari 2MRTU 960, aripiprazole 2-month ready-to-use 960 mg; CGI-BP, Clinical Global Impression – Bipolar Version; LS, least squares; MADRS, Montgomery–Åsberg Depression Rating Scale; MMRM, mixed model for repeated measures; SD, standard deviation; SE, standard error; SWN-S, Subjective Well-being under Neuroleptic Treatment – Short Form; YMRS, Young Mania Rating Scale.
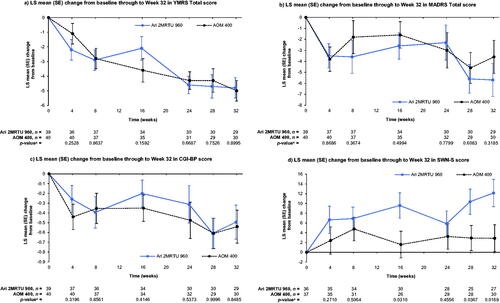
Table 3. Efficacy outcomes at Week 32 in patients with BP-I.
McIntyre_et_al._Supplementary_material_5-May-23.pdf
Download PDF (361.9 KB)D ata availability statement
To submit inquiries related to Otsuka clinical research, or to request access to individual participant data (IPD) associated with any Otsuka clinical trial, please visit https://clinical-trials.otsuka.com/. For all approved IPD access requests, Otsuka will share anonymized IPD on a remotely accessible data sharing platform.
