Figures & data
Table 1. MEDLINE database search strategy.
Figure 1. PRISMA flow diagram for the selection process of the references retrieved by the MEDLINE search. Abbreviations. COA, clinical outcome assessments; PRO, patient reported outcomes
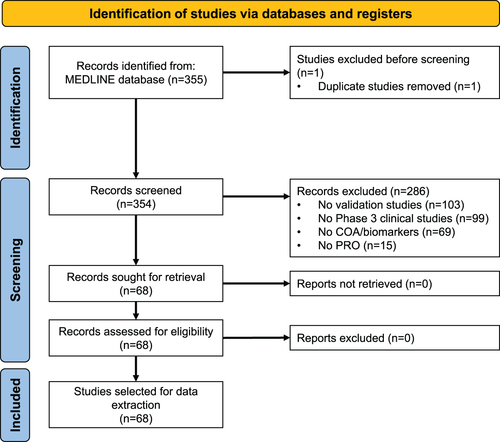
Figure 2. Labelling claims flow diagram. aData on study design, therapeutic indication, and psychometric assessment results extracted from 21 studies for all the 20 instruments validated in the publications. Abbreviations. OMDQ, Oral Mucositis Daily Questionnaire; PEP, Premature Ejaculation Profile; PDQ, Peyronie’s Disease Questionnaire; PRO, patient reported outcome. WPS-RA, Rheumatoid arthritis-specific Work Productivity Survey; VVSymQ, Varicose Veins Symptoms Questionnaire.
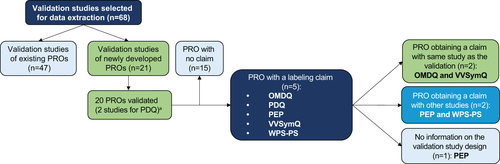
Table 2. PROs with label claim.
5055_PRO_TLR_ms_Supplementary_material_26May23_v1.0__clean_.docx
Download MS Word (521 KB)Data availability statement
Researchers may request access to anonymized participant-level data, trial-level data and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
