Figures & data
Figure 1. Patient attrition. Abbreviations. AEMR, IQVIA’s Ambulatory Electronic Medical Record database; LRx, IQVIA’s prescription claims database; HIV, human immunodeficiency virus; INSTI, integrase strand inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; RNA, ribonucleic acid. aAEMR prescription data were prioritized when prescription claims in LRx and prescription records in AEMR were for the same medication (identified by the National Drug Code [NDC]) with the same days of supply on the same date. This was performed to avoid overestimation of treatment duration when the LRx and AEMR records were suspected to correspond to the same prescription. bData cleaning steps were taken to remove clinically implausible weight measurements (e.g. weight measurement that is beyond [previous measurement – 20 kg, previous measurement + 20 kg]). cConcurrent treatment was defined as ≥90-day overlap in days of supply for two different anchor agent classes or evidence of a fixed-dose combination medication containing two different anchor agents (e.g. NNRTI and INSTI). dMalignancy, pregnancy, and HIV-2 were identified using diagnosis codes. Gastric bypass was identified using procedure codes and diagnosis codes.
![Figure 1. Patient attrition. Abbreviations. AEMR, IQVIA’s Ambulatory Electronic Medical Record database; LRx, IQVIA’s prescription claims database; HIV, human immunodeficiency virus; INSTI, integrase strand inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; RNA, ribonucleic acid. aAEMR prescription data were prioritized when prescription claims in LRx and prescription records in AEMR were for the same medication (identified by the National Drug Code [NDC]) with the same days of supply on the same date. This was performed to avoid overestimation of treatment duration when the LRx and AEMR records were suspected to correspond to the same prescription. bData cleaning steps were taken to remove clinically implausible weight measurements (e.g. weight measurement that is beyond [previous measurement – 20 kg, previous measurement + 20 kg]). cConcurrent treatment was defined as ≥90-day overlap in days of supply for two different anchor agent classes or evidence of a fixed-dose combination medication containing two different anchor agents (e.g. NNRTI and INSTI). dMalignancy, pregnancy, and HIV-2 were identified using diagnosis codes. Gastric bypass was identified using procedure codes and diagnosis codes.](/cms/asset/769038e6-eec0-4f62-8e6e-c35ca6d6c9ab/icmo_a_2224165_f0001_b.jpg)
Table 1. Demographic clinical characteristics of the NNRTI, PI, and INSTI cohorts.
Table 2. Baseline clinical characteristics of the NNRTI, PI, and INSTI cohorts.
Figure 2. Unadjusted changes in weight from baseline to follow-up for the NNRTI, PI, and INSTI cohorts. Abbreviations. ART, antiretroviral therapy; INSTI, integrase strand inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor. The comparison of mean weight change from baseline across the NNRTI, PI, and INSTI cohorts was significantly different (p <.05) at all timepoints.
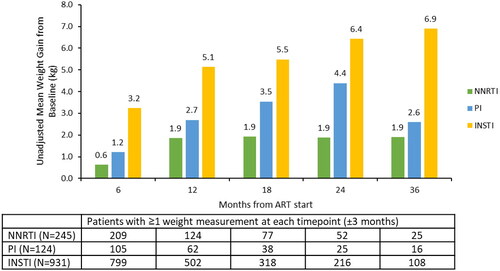
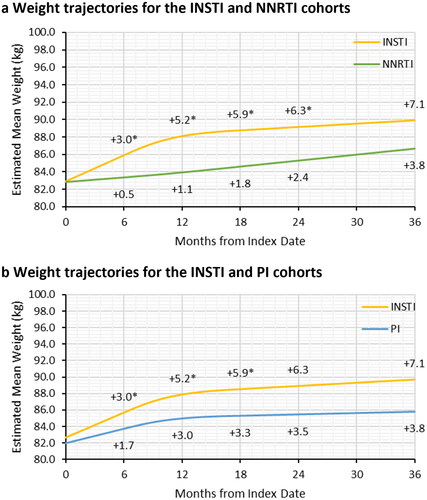
Figure 3. Adjusted weight trajectories after initiation of NNRTI-based ART (a) and PI-based ART (b) compared to INSTI-based ART. Abbreviations. ART, antiretroviral therapy; INSTI, integrase strand inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor. *Significant difference between the INSTI and NNRTI or PI cohorts in gained weight relative to the baseline weight at the specified follow-up time. p-values and estimated mean weight were obtained from multivariable linear mixed model with random intercept and restricted cubic splines with three knots to adjusted for non-linear trend of weight change trajectory. The first knot was placed at 6 months of follow-up and the remaining two knots were placed at median and third quartile of follow-up. The models adjusted for age at index, sex, race/ethnicity, Charlson comorbidity index, payer type, evidence of baseline anxiety or depression, and evidence of baseline diabetes or dyslipidemia/hyperlipidemia.
Supplemental_materials.docx
Download MS Word (876.6 KB)Data availability statement
The data that support the findings of this study are available from IQVIA but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
