Figures & data

Figure 1. Network of studies for ETHOS and IMPACT.
BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; FF, fluticasone furoate; GFF, glycopyrrolate/formoterol fumarate; ITC, indirect treatment comparison; MAIC, matching-indirect treatment comparison; UMEC, umeclidinium; VI, vilanterol
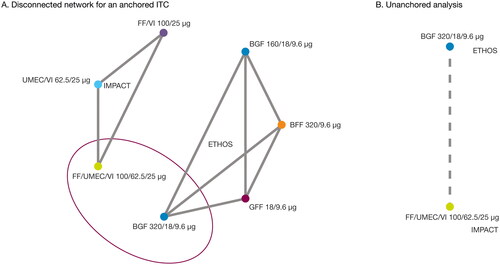
Table 1. Summary of primary MAIC and sensitivity analyses.
Figure 2. Kaplan–meier curves and hazard ratios for all-cause mortality (MAICa,b and unadjusted analyses) for BGF from ETHOS versus FF/UMEC/VI from IMPACT over 52 weeks (ITT populationc).
aBGF was adjusted according to aggregate FF/UMEC/VI data from IMPACT for sex, body mass index, smoking status, race (Asian, White, Other), severe exacerbation history in the last 12 months, bronchodilator reversibility, and COPD severity (moderate, severe, very severe).
bIn the MAIC analysis, absolute risk reduction of BGF versus FF/UMEC/VI at week 52 was 0.009 (95% CI [Greenwood SE]: 0.0016, 0.0164), corresponding to a number needed to treat of 112. MAIC weights were scaled to the original sample size when computing the SE so that they are representative of the quantity of data available.
cBoth analyses used on- and off-treatment data in the final retrieved data, which included 99.6% of data from the ITT populations of both ETHOS and IMPACT.
BGF, budesonide/glycopyrrolate/formoterol fumarate 320/18/9.6 μg; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FF/UMEC/VI, fluticasone furoate/umeclidinium/vilanterol 100/62.5/25 μg; HR, hazard ratio; ITT, intention-to-treat; MAIC, matching-adjusted indirect comparison; SE, standard error.
![Figure 2. Kaplan–meier curves and hazard ratios for all-cause mortality (MAICa,b and unadjusted analyses) for BGF from ETHOS versus FF/UMEC/VI from IMPACT over 52 weeks (ITT populationc).aBGF was adjusted according to aggregate FF/UMEC/VI data from IMPACT for sex, body mass index, smoking status, race (Asian, White, Other), severe exacerbation history in the last 12 months, bronchodilator reversibility, and COPD severity (moderate, severe, very severe).bIn the MAIC analysis, absolute risk reduction of BGF versus FF/UMEC/VI at week 52 was 0.009 (95% CI [Greenwood SE]: 0.0016, 0.0164), corresponding to a number needed to treat of 112. MAIC weights were scaled to the original sample size when computing the SE so that they are representative of the quantity of data available.cBoth analyses used on- and off-treatment data in the final retrieved data, which included 99.6% of data from the ITT populations of both ETHOS and IMPACT.BGF, budesonide/glycopyrrolate/formoterol fumarate 320/18/9.6 μg; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FF/UMEC/VI, fluticasone furoate/umeclidinium/vilanterol 100/62.5/25 μg; HR, hazard ratio; ITT, intention-to-treat; MAIC, matching-adjusted indirect comparison; SE, standard error.](/cms/asset/8aeaf035-fe21-4fd4-8472-313912ea97bb/icmo_a_2247969_f0002_c.jpg)
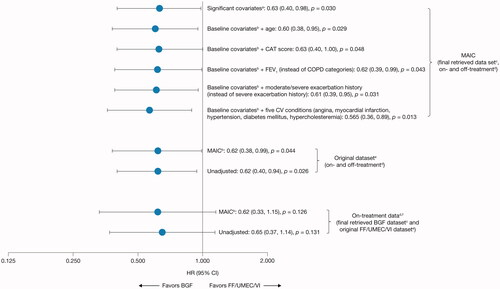
Figure 3. Hazard ratios for all-cause mortality (MAIC and unadjusted sensitivity analyses) for BGF from ETHOS versus FF/UMEC/VI from IMPACT (ITT population).
aSignificant covariates (as measured by a standardized mean difference >0.1): sex, body mass index, bronchodilator reversibility, and COPD severity category (moderate, severe, very severe).
bBGF was adjusted according to aggregate FF/UMEC/VI data from IMPACT for sex, race (Asian, White, Other), body mass index, smoking status, severe exacerbation history in the last 12 months, bronchodilator reversibility, and COPD severity (moderate, severe, very severe).
cThe final retrieved datasets included the original datasets plus additional data retrieved for patients missing Week 52 vital status, resulting in data for 99.6% of the ITT populations for each study.
dA death was defined as “on-treatment” in IMPACT if the date of death occurred ≤7 days after the last treatment day and was considered “off-treatment” if the date of death occurred >7 days after the last treatment day and up to within 7 days of the projected Week 52 dateCitation24. In ETHOS, time to all-cause death was a prespecified secondary endpoint and was assessed in the ITT population using the treatment policy estimand, which included all randomized patients who received any amount of study drug and all observed data within 52 weeks of randomization regardless of whether patients remained on randomized treatmentCitation23. Data from within 52 weeks of randomization was used for the on- and off-treatment analyses.
eDataset established at database lock.
fA 7-day data cut-off from ETHOS for on-treatment sensitivity analysis was used to be consistent with the definition in IMPACT.
BGF, budesonide/glycopyrrolate/formoterol fumarate 320/18/9.6 μg; CAT, COPD Assessment Test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FF/UMEC/VI, fluticasone furoate/umeclidinium/vilanterol 100/62.5/25 μg; HR, hazard ratio; ITT, intention-to-treat; MAIC, matching-adjusted indirect comparison.
Supplemental Material
Download MS Word (342.1 KB)Data availability statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/.
