Figures & data
Table 1. Patient demographics and disease characteristics.
Table 2. Adjusted completion rates for HRQoL assessments.
Figure 1. EORTC QLQ-C30 Mean change from baseline in GHS and functioning scales at cycle 7 (6 months) by treatment. Abbreviations. CI, confidence interval; EMTD, estimated mean treatment difference; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Core 30; GHS, global health status.
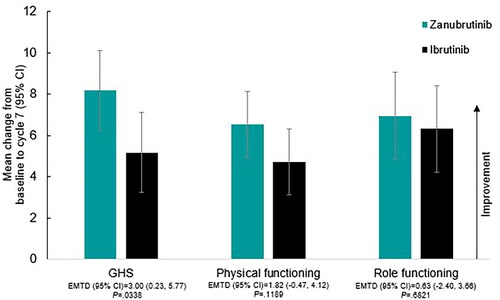
Figure 2. EORTC QLQ-C30 Mean change from baseline in GHS and functioning scales at cycle 13 (12 months) by treatment. Abbreviations. CI, confidence interval; EMTD, estimated mean treatment difference; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Core 30; GHS, global health status.
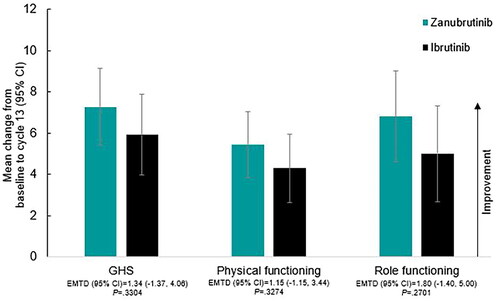
Figure 3. EORTC QLQ-C30 Mean change from baseline in symptom scales at cycle 7 (6 months) by treatment. Abbreviations. CI, confidence interval; EMTD, estimated mean treatment difference; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Core 30.
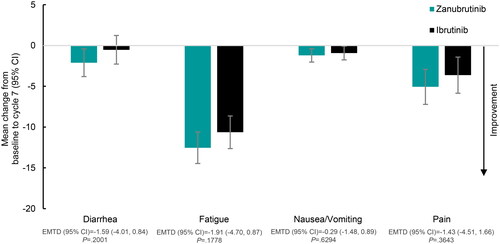
Figure 4. EORTC QLQ-C30 Mean change from baseline in symptom scales at cycle 13 (12 months) by treatment. Abbreviations. CI, confidence interval; EMTD, estimated mean treatment difference; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Core 30.
Supplemental Material
Download MS Word (22.5 KB)Data availability statement
BeiGene voluntarily shares anonymous data on completed studies responsibly and provides qualified scientific and medical researchers access to anonymous data and supporting clinical trial documentation for clinical trials in dossiers for medicines and indications after submission and approval in the United States, China, and Europe. Clinical trials supporting subsequent local approvals, new indications, or combination products are eligible for sharing once corresponding regulatory approvals are achieved. BeiGene shares data only when permitted by applicable data privacy and security laws and regulations. In addition, data can only be shared when it is feasible to do so without compromising the privacy of study participants. Qualified researchers may submit data requests/research proposals for BeiGene review and consideration through BeiGene’s Clinical Trial Webpage at https://www.beigene.com/our-science-and-medicines/our-clinical-trials/.
