Figures & data
Table 1. Patient characteristics at the time of the survey.
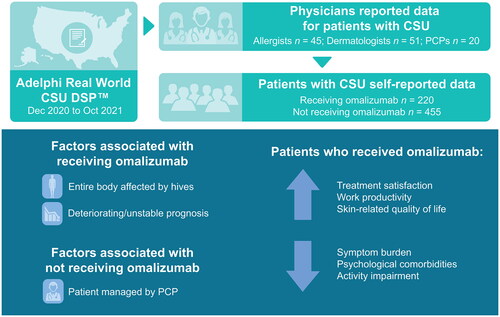
Figure 1. Factors associated with receiving omalizumab (OMA; n = 760; n = 242 receiving OMA; n = 518 not receiving OMA) were identified by logistic regression (odds ratios [OR] and 95% confidence intervals [CI] are presented). An OR >1 indicates that the factor is associated with receiving OMA, whereas an OR <1 indicates that the factor is not associated with receiving OMA. With the exception of age and number of symptoms present, all other ORs are defined in comparison to a reference group: physician specialty general practitioner (GP)/primary care physician (PCP) or dermatologist (compared with allergist); experiencing a symptomatic period (compared with not experiencing a symptomatic period); male (compared with female); experiencing depression (compared with no depression); experiencing anxiety/distress (compared with no anxiety/distress); severe/extremely severe insomnia (compared with none/mild/moderate insomnia); intense hives (>50 hives/24 h) (compared with none/mild/moderate hives [≤50 hives/24 h]); visible areas affected, including hands/fingers, face, head, and neck (compared with no visible areas affected); severe/extremely severe itch (compared with none/mild/moderate itch); experiencing an angioedema episode (compared with not experiencing an angioedema episode); experiencing severe chronic spontaneous urticaria (CSU; compared with moderate CSU); deteriorating/unstable progression of CSU (compared with improving/stable progression); and entire body affected by hives/itching (compared with not entire body). All patients were physician-categorized as having moderate or severe CSU at the time of current treatment initiation. *p < 0.05 receiving OMA vs. not receiving OMA.
![Figure 1. Factors associated with receiving omalizumab (OMA; n = 760; n = 242 receiving OMA; n = 518 not receiving OMA) were identified by logistic regression (odds ratios [OR] and 95% confidence intervals [CI] are presented). An OR >1 indicates that the factor is associated with receiving OMA, whereas an OR <1 indicates that the factor is not associated with receiving OMA. With the exception of age and number of symptoms present, all other ORs are defined in comparison to a reference group: physician specialty general practitioner (GP)/primary care physician (PCP) or dermatologist (compared with allergist); experiencing a symptomatic period (compared with not experiencing a symptomatic period); male (compared with female); experiencing depression (compared with no depression); experiencing anxiety/distress (compared with no anxiety/distress); severe/extremely severe insomnia (compared with none/mild/moderate insomnia); intense hives (>50 hives/24 h) (compared with none/mild/moderate hives [≤50 hives/24 h]); visible areas affected, including hands/fingers, face, head, and neck (compared with no visible areas affected); severe/extremely severe itch (compared with none/mild/moderate itch); experiencing an angioedema episode (compared with not experiencing an angioedema episode); experiencing severe chronic spontaneous urticaria (CSU; compared with moderate CSU); deteriorating/unstable progression of CSU (compared with improving/stable progression); and entire body affected by hives/itching (compared with not entire body). All patients were physician-categorized as having moderate or severe CSU at the time of current treatment initiation. *p < 0.05 receiving OMA vs. not receiving OMA.](/cms/asset/f71117a1-84e8-4be8-94d3-7573e381f1ce/icmo_a_2354534_f0001_c.jpg)
Figure 2. Physician-reported symptoms in patients receiving and not receiving omalizumab (OMA), as estimated using an inverse probability weighted regression adjustment model (n = 675; n = 220 receiving OMA; n = 455 not receiving OMA). aSince initiation of current treatment. Abbreviations. ATE, average treatment effect; NA, not available; SE, standard error.
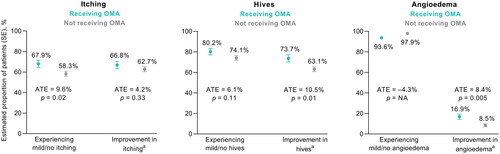
Figure 3. Sleep and psychological outcomes in patients receiving and not receiving omalizumab (OMA), as estimated using an inverse probability weighted regression adjustment model (n = 675; n = 220 receiving OMA; n = 455 not receiving OMA). aSince initiation of current treatment. Abbreviations. ATE, average treatment effect; SE, standard error.
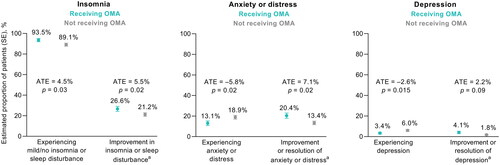
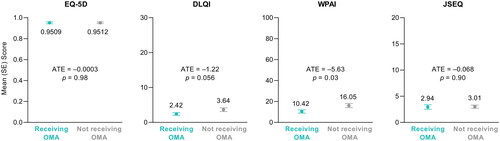
Figure 4. Patient-reported quality of life outcomes in patients receiving and not receiving omalizumab (OMA), as estimated using an inverse probability weighted regression adjustment model (n = 188; n = 60 receiving OMA; n = 128 not receiving OMA). Abbreviations. ATE, average treatment effect; DLQI, Dermatology Life Quality Index; JSEQ, Jenkins Sleep Evaluation Questionnaire; SE, standard error; WPAI, Work Productivity and Activity Impairment.