Figures & data
Figure 1. Study flow-chart. NSCLC: non-small cell lung cancer; eCRFs: electronic case report form; RECIST: Response evaluation criteria in solid tumors; OS: overall survival; PFS: progression-free survival; 2L: second-line, 3L/LL: third-line or later-line.
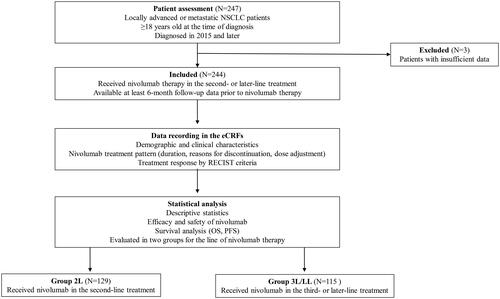
Table 1. Demographic and clinical characteristics of patients.
Table 2. Treatment-related information of the whole study population (N = 244).
Table 3. Comparison of demographic and clinical characteristics of the patients in the treatment line groups.
Table 4. Comparison of molecular characteristics (driver mutations) of the patients in the treatment line groups.
Figure 2. (a) Overall survival and (b) Progression-free survival curve for the whole study population.
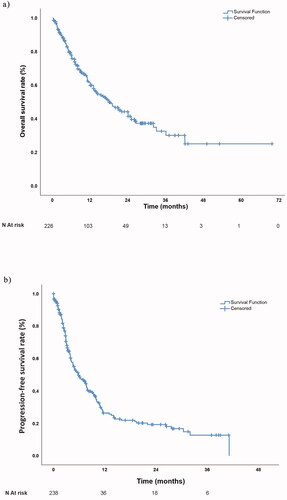
Table 5. Treatment response rates by RECIST criteria according to the treatment line groups.
Table 6. Frequency of adverse events according to the treatment line groups.
Table 7. Reasons for discontinuation of nivolumab therapy.
