Figures & data
Figure 1. Enrolled patients with acute bronchitis. Abbreviations. AGS, investigational drug (Synatura syrup); AGU, comparative drug (Umckamin); FAS, full analysis set; PPS, per-protocol set.
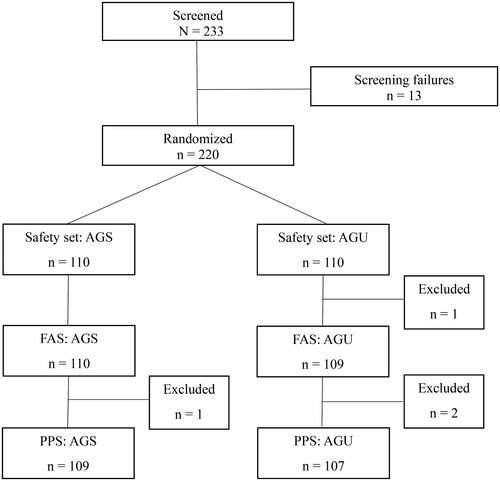
Table 1. Baseline characteristics of randomized patients.
Figure 2. Change of the total bronchitis severity score (BSS) of the AGS and AGU groups from the baseline visit (visit 2) to end of treatment visit (visit 3) in the per-protocol set (A) and full analysis set (B). Abbreviations. BSS, bronchitis severity score; AGS, investigational drug (Synatura syrup); AGU, comparative drug (Umckamin).
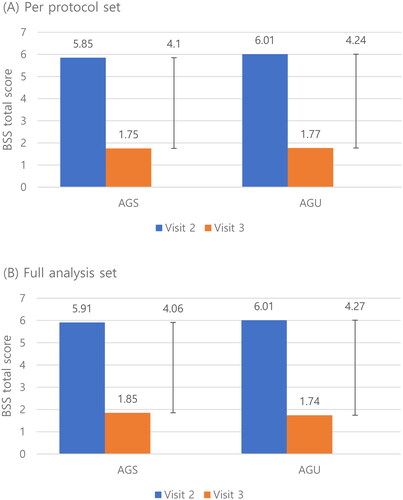
Figure 3. Mean difference in the total BSS between visits 2 and 3 in per-protocol analysis. Abbreviation. BSS, bronchitis severity score.

Table 2. Difference in each BSS component of the AGS and AGU groups with treatment.
Table 3. Treatment response rate between visits 2 and 3.
Table 4. Treatment response rate between visits 2 and 3.
Table 5. Subjective satisfaction of patients.
Table 6. Adverse events during the clinical trial.
