Figures & data
Figure 1. Schematic overview of three scenarios and their data sources.
1L, first line; 2L, second line; CT/CIT, chemotherapy/chemoimmunotherapy.
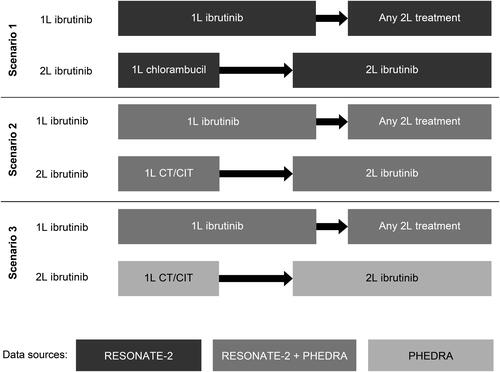
Table 1. Baseline characteristics for cohorts of Scenarios 1, 2, and 3.
Table 2. Treatment regimens for cohorts of Scenarios 1, 2, and 3.
Figure 2. Kaplan–Meier curves for Scenario 1 (A), Scenario 2 (B), and Scenario 3 (C). Overall survival analysis of observed data and adjusted data using the weighted Cox PH model.
1L, first line; 2L, second line; CT/CIT, chemotherapy/chemoimmunotherapy; CI, confidence interval; HR, hazard ratio; PH, proportional hazards.
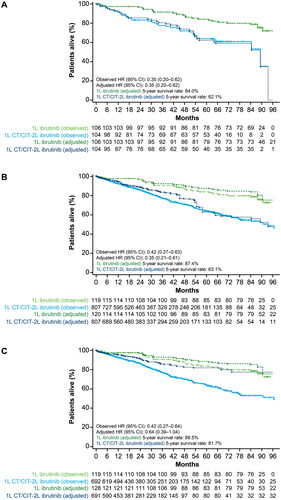
Table 3. Summary of observed and adjusted overall survival.
Data availability statement
Data used for this study were based on the RESONATE-2 study and PHEDRA database. PHEDRA is a non-interventional, secondary-use project which collaborates with the owners of existing databases of electronic health records in the Europe, Middle East, and Africa (EMEA) region and gathers deidentified patient-level data on CLL, mantle cell lymphoma, and Waldenström macroglobulinemia centrally. The real-world databases utilized in this study are not owned by Janssen Pharmaceutica NV. The Lyon-Sud data included in this study are based on patient-level information collected by Lyon-Sud University Hospital in the French routine care setting. The CLLEAR data included in this study are based on patient-level information collected by contributing local hospitals at seven academic centers in the Czech Republic, located in Brno, Hradec Králové, Nový Jičín, Olomouc, Ostrava, Plzeň, and Prague. Requests for access to data from Lyon-Sud University Hospital and the CLLEAR registry should be sent directly to the respective owners of the databases. RESONATE-2 data sharing is governed by Pharmacyclics LLC, an AbbVie Company. Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
