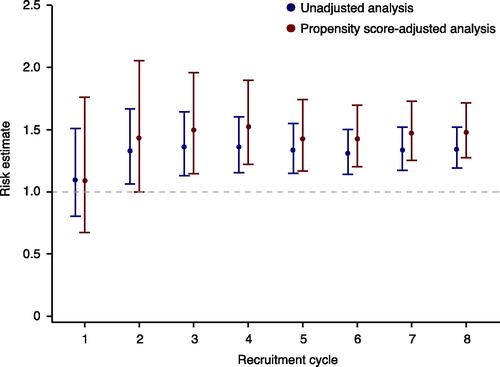
Figures & data
Figure 1. In each recruitment cycle, new users of drug A and new users of drug B are included and added to the cohort to continuously increase the study sample size.
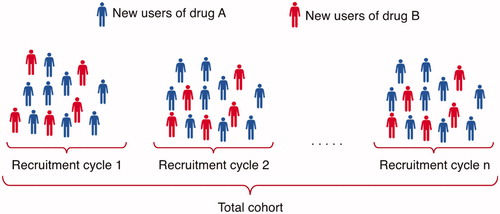
Figure 3. Standardized differences in baseline covariates between new users of drug A and new users of drug B before and after adjusting on the propensity score. A standardized difference <0.1 indicates negligible imbalance.
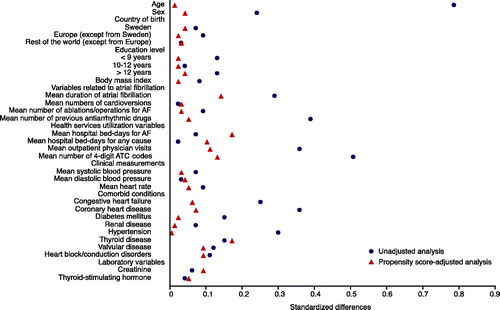
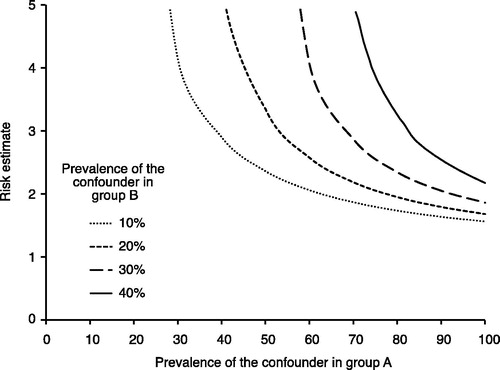
Figure 4. Evaluation of how powerful an unmeasured confounder would have to be to change the observed results. For example, if the prevalence of a potential unmeasured confounder is 40% in the drug A group (x-axis) and 10% in the drug B group, then the unmeasured confounder must have a risk estimate (hazard ratio) of the outcome close to 3 to fully explain the advantage of drug A over drug B.